
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The temperature at state A is 20ºC, that is 293 K, what is the temperature at state B?
|
40ºC |
||
|
10ºC |
||
|
146.5 K |
||
|
not enough information |
||
|
586 K |
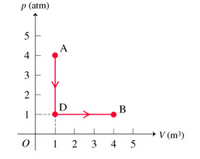
Transcribed Image Text:This diagram is a pressure-volume (P-V) graph illustrating the relationship between pressure \( p \) in atmospheres (atm) and volume \( V \) in cubic meters (m³) for a thermodynamic process involving a system.
### Graph Details:
- **Axes:**
- The vertical axis represents the pressure \( p \) in atmospheres (atm), marked from 0 to 5.
- The horizontal axis represents the volume \( V \) in cubic meters (m³), marked from 0 to 5.
- **Points and Process Path:**
- **Point A:** Located at 1 m³ and 4 atm.
- **Point B:** Located at 4 m³ and 1 atm.
- **Point D:** Located at 1 m³ and 1 atm.
- **Process Description:**
- The system starts at point A, moving vertically downward to point D while maintaining a constant volume of 1 m³ as the pressure decreases from 4 atm to 1 atm.
- From point D, the system moves horizontally to point B while maintaining a constant pressure of 1 atm as the volume increases from 1 m³ to 4 m³.
The red arrows indicate the direction of the process flow, demonstrating a two-step thermodynamic process involving a decrease in pressure followed by an increase in volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What is the temperature change of 10 K in Fahrenheit? A: 18 B: 8 C: 20 D: 10 E: 15arrow_forwardQUESTION 14 A balloon contains 0.300 m3 of Helium at a pressure of 1.100 x 10° Pa. The temperature of the helium is 310 K. What is the mass (in kg) is the helium in the balloon?arrow_forwardA piece of copper of mass 2kg is cooled from 150°C to 50°C. The specific heat capacity of copper is 400 J/ kg °C. The heat loss is Oa. 4000 J b. 80000 J Oc. 40000 J O d. 800 Jarrow_forward
- An aluminum plate has a circular hole. If the temperature of the plate increases, what happens to the size of the hole? 7.jpg Increases Decreases Stays the same Increases the top half of the hole More information is requiredarrow_forward10. If it takes 554 calories to increase the temperature of 100 grams of a substance by 60.0 degrees Celsius then how many calories does it take to raise the temperature of 600 grams of the same substance by 60.0 degrees Celsius? No phase changes occur. a. 13300 b. 554 c. 3324arrow_forward2900000 48 cal/g eat meat sheet of paper, solve the following problems. Note that quantity of heat energy required to change the phase of a substance is given by the following equation: Q = mL, where is the heat required, m is the mass, and L is the heat of fusion or vaporization. 1. How much energy is absorbed when 25.0 g of ice at 0.0°C melts to form water at 0.0°C? The heat of fusion of ice is 80 cal/g. 2. How much energy is absorbed when the water at 0.0°C in Question 1 is heated to 100.0°C? The specific heat of water is 1.0 cal/g °C. 3. How much energy is absorbed when the water at 100.0°C in Question 2 vaporizes to 100.0°C? The heat of vaporization of water is 540 cal/g. 4. The steam in Question 3 is used to melt ice. How much ice will be melted by the energy released by the condensation of the steam at 100.0°C?arrow_forward
- 5 In order to determine the heat capacity of their latest phone, CrabappleTM engineers cool the phone in the freezer to -31.7ºC. They then drop it into a calorimeter with some water in it that is at a temperature of 50.8ºC. The calorimeter (including the water) has a total heat capacity of 869.42 J/ºC. After a few minutes the temperature of the calorimeter is stabilized to 31ºC. What is the heat capacity of the phone? Enter your answer with at least 3 sig figs assuming units of J/ºC.arrow_forwardProblem 6: Suppose you pour 0.0125 kg of 20.0°C water onto a 1.1-kg block of ice, sitting in a large bowl, which is initially at -15.0°C. The latent heat of fusion for water is Lf = 334 kJ/kg. What is the final temperature of this system? You may assume that the water cools so rapidly that effects of the surroundings are negligible.arrow_forward1) Listen How much energy would have to be added to 519grams of water to heat it from 17.0°C to 84.0°C? O 3.53x105 J O 1.46x108 J O 6.95x104 J O7.39x105 J O7.39x108 J O 1.46x105 Jarrow_forward
- €2 When an air bubble rises from the bottom to the top of a freshwater lake, its volume increases by 75%. If the temperature at the bottom of the lake was 3°C and at the top was 13.5°C, how deep is the lake? Hint The lake is Question Help: Message instructor Submit Question #3 3 E 80 F3 $ 4 F4 R cr dº % m deep. 5 F5 T A 6 MacBook Air F6 & 7 F7 Y U *00 8 DII F8 - ( 9 DD F9 1 0 0arrow_forwardA copper sample (ccu = 387 J/kg•K) of mass mẹ = 75 g and temperature Te = 312ºC is dropped into a glass beaker that contains a mass of water me = 220 g (cw = 4190 J/kg•K). %3D The initial temperature of the water and the beaker is Tw,b = 12.0°C. What is the final temperature of the copper, beaker, and water? * The heat capacity (the specific heat times the mass) of the beaker is Cb' = 190 J/K.arrow_forwardQUESTION 7 The average body temperature of a living human being is 98.6° F. This temperature in kelvins is а. 300 K b. 310 K C. 293 K d. 273 K е. 283 Кarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON