
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The process abc shown in the pV-diagram in Fig.
involves
0.0175 mol of an ideal gas. (a) What was the lowest temperature
the gas reached in this process? Where did it occur? (b) How much
work was done by or on the gas from a to b? From b to c? (c) If 215 J
of heat was put into the gas during abc, how many of those joules went
into internal energy?
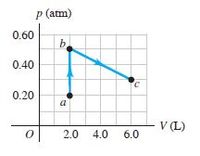
Transcribed Image Text:P (atm)
0.60
b.
0.40
0.20
a
V (L)
2.0 4.0 6.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A four-liter tank is filled with propane gas, C3H8. The mass of the tank filled with gas is 1236 g. The pressure in the tank is 2.68 atm. The temperature in the room is 37C. The propane in the tank is used up under the same conditions of temperature and pressure. What is the mass of the empty tank?arrow_forwardVolume of HCI Added (mL) 0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Pressure (atm) 1.000 1.059 1.118 1.178 1.238 1.298 1.355 1.359 1.362 Volume of Gas (mL) 214.0 213.5 213.0 212.5 212.0 211.5 211.0 210.5 210.0arrow_forwardCPAP is the acronym for "Continuous Positive Airway Pressure". A CPAP device is used in respiratory therapy. Some ambulances carry a CPAP device. The pressure unit used in CPAP devices is "cm H2O". A typical pressure setting on a CPAP device may be 9 cm H2O. Blood pressure is commonly measured in units of mm Hg (or millimeters mercury). Which of the following statements is true regarding the pressure of 1 cm H2O ? Question 17 options: A) 1 in H2O has a lesser pressure than 1 cm H2O. B) 1 in H2O has a greater pressure than 1 cm H2O. C) 1 mm Hg has a lesser pressure than 1 cm H2O. D) None of the above answer choices are correct.arrow_forward
- A cylinder is filled with 10.0 L of gas and a piston is put into it. The initial pressure of the gas is measured to be 113. kPa. The piston is now pulled up, expanding the gas, until the gas has a final volume of 20.0 L. Calculate the final pressure of the gas. Be sure your answer haş the correct number of significant digits. piston cylinder gas olo Ar ||kPa x10arrow_forwardComplete the highlighted section Darrow_forwardComplete the following scheme 13 2 00 ? 1. LDA 2. 101 (CH,) NH DCC 2arrow_forward
- A cylinder is filled with 10.0 L of gas and a piston is put into it. The initial pressure of the gas is measured to be 175. kPa. The piston is now pulled up, expanding the gas, until the gas has a final volume of 64.0 L. Calculate the final pressure of the gas. Be sure your answer has the correct number of significant digits. piston olo cylinder gas Ar ||kPa x10arrow_forwardStandard temperature and pressure are (1atm=760mmHg)arrow_forward●●● 6 15 16 Keynote File Edit Insert Slide Format Arrange View Play ... 17 18 19 20 21 ✓ View 100% Zoom :0 F1 + Add Slide V View 125% Zoom ► Play + Add Page Keynote Live F2 O 38,742 Table 20 T Insert F3 Share Window Help APR 13 000 000 6 Table GAS LAWS HOMEWORK ASSIGNMENT (You can examine the data on the Student Data Sheets for assistance.) F4 GASES LAB HW (1) ~ GASES LAB Chart . Chart T Text 1.The pressure of a gas is doubled. Assuming that n and T are constant, what should happen to the volume of the gas? T Text 2.The pressure of a gas is doubled. Assuming that n and V are constant, what should happen to the temperature of the gas? Shape 3.The number of moles of gas is doubled. Assuming P and T are constant, what should happen to the volume of the gas? F5 P Media Shape density PA Media Comment F6 MacBook Air ◄◄ Comment F7 No Collaborate Color Fill Appearance Title Body [Slide Number ▾ Background ▶11 4 Collab F8 ● For Sli Edit Marrow_forward
- 12. (c) Potassium metal reacts with water. 2K(s) + 2H,0(1) → 2KOH(aq) + H,(g) 0.2346g of potassium is reacted with excess water. Calculate the volume of gas formed. The gas volume is measured in cm° at room temperature and pressure. answer =arrow_forwardWhat is STP? standard theoretical measurements standard values for temperature and pressure O symbols of pure compounds standard technologies used in experimentsarrow_forward3. At constant pressure, 8.0 liters of a gas is heated from 25.5°C to 50°C. What is the new volume of the gas?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning