
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give typed explanation
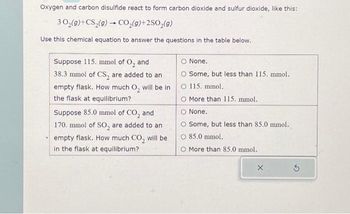
Transcribed Image Text:Oxygen and carbon disulfide react to form carbon dioxide and sulfur dioxide, like this:
30₂(g) +CS₂(g) → CO₂(g)+2SO₂(g)
Use this chemical equation to answer the questions in the table below.
Suppose 115. mmol of O₂ and
38.3 mmol of CS, are added to an
empty flask. How much O₂ will be in
the flask at equilibrium?
Suppose 85.0 mmol of CO₂ and
170. mmol of SO₂ are added to an
empty flask. How much CO₂ will be
in the flask at equilibrium?
O None.
O Some, but less than 115. mmol.
O 115. mmol.
O More than 115. mmol.
O None.
Some, but less than 85.0 mmol.
O 85.0 mmol.
More than 85.0 mmol.
X
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- write a chemical reaction scheme for the synthesis of salicylic acid from wintergreen based on the information provided below. Include the balanced chemical equation for the reaction being studied or conducted (reactants to products). indicate actual chemical structures that pertain to the experiment and indicate conditions as well as solvents over and under the reaction arrow.arrow_forwardс www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3JH-IQUHIQg6bJxmeSyVPHOEB1plef9xy C5Ca9QIUX9FDNs1kHQvOMzcrVpгA IBP5q2HYwPcASZQKNla... Naming and Drawing Organic Molecules Recognizing different skeletal structures How many different molecules are drawn below? mxxx sex Explanation Check G MacBook Air 0/5 Julianna ? olo Ar 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardCan someone please helparrow_forward
- Organic Chem 1 Week 5 Lab- Student.docx - Saved to this PC P Search shiku24 Design Layout References Mailings Review View Developer Help A A Aa v Ao E - E MaBbCcDd AaBbCc Dd AaBbCc AaBbCcD AaBi 1 Normal 1 No Spac. Heading 1 * A. P. A 三。、。 Reola Heading 2 T'tle > Select Paragraph Stylrs Editing 4. Prodiet the producti s) of the lollowing reactions, including stereochemistry when necessury und identify the mechanism of each substitution eaction (Syl vs SN2). product(s) type of reaction Br NaSMe DMSO Nal acetone HO Toxt Predicliong: On Accesiby. Irvestigate Enylish (United States) (99+ o search Pr ** FI1 FS & %23 6 7 2 3 R T. K L J F G C V Alt Alt LEarrow_forwardHey!! For my science class I need to present a scienfic paper. I don't know how to find scienfic papers on the internet. If you could give me a quick couple to sentences explation of how to search of research papers on the web (SCIENFIC PAPERS NOT JUST ARTICLES/NEWS REPORTS) that would be serouisly help me a lot. I know this isn't your typically question, but this would seriously mean the world to me. Thanksarrow_forwardInterface/acellus_engine.html?ClassID=845449353 How many moles of Al are present in 1 mole of Al₂S3 ? moles Al Ernational Academy of Science. All Rights Reserved. Ri 5 A 6 Moles Al O 2 & 7 PrtScn 8 Enter Homearrow_forward
- Please help me, the lesson name : making use of synthetic polymers.arrow_forwardwhat is product LiAlH4,Et2Oarrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
- This is not really a question but, I have a presentation to do on one of the following polymers. I was just wondering if you can pick the top 5 best out of them all. I will include the questions I will be researching, I just want an expert opinion on which polymer would be th best for these questions. :) These are the questions that I will be resaerching about: List its current uses and why we use it for those applications. What is it? What are its components (i.e., its monomers)? How is the polymer manufactured? What is the chemical reaction that occurs in its creation? What is the chemical reaction that occurs in its degradation? What are the environmental consequences of its production? What are the environmental consequences of its disposal? Propose a solution to the environmental consequences you listed above. For example, could something about the polymer be changed to alleviate the problems or could another, safer polymer be used in its place? And these are the polymers I…arrow_forwardThe name carbohydrate comes from the fact that many simple sugars have chemical formulae that look like water has simply been added to carbon. (The suffix hydrate from the Greek word hydor ("water") means "compound formed by the addition of water.") The actual chemical structure of carbohydrates doesn't look anything like water molecules bonded to carbon atoms (see sketch at right). But it is nevertheless possible to chemically extract all the hydrogen and oxygen from many simple carbohydrates as water, leaving only carbon behind. If you search the Internet for "reaction of sulfuric acid and sugar" you will find some impressive videos of this. Suppose you had 300.g of ordinary table sugar, which chemists call sucrose, and which has the chemical formula C12H22O11 . Calculate the maximum mass of water you could theoretically extract. Be sure your answer has a unit symbol, and round it to the correct number of significant digits.arrow_forwardFile Edit View History Bookmarks Profiles Tab Window Help Chrome dt8827@unc X aModule 11 F X My Wellness x A ALEKS - Day x A ALEKS - Re Watch Gilmo x A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-lvdWKW_BBZZI6tTytly4Fcfu6zOtOf8oMM9svvsLU ps Spotify Web Playe. M Common Ethical D. O CHEMICAL REACTIONS Interconverting number of atoms and mass of compound Calculate the number of oxygen atoms in a 120.0 g sample of diphosphorus pentoxide (P,O,). Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits. Explanation Check © 202 APR 18arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY