
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
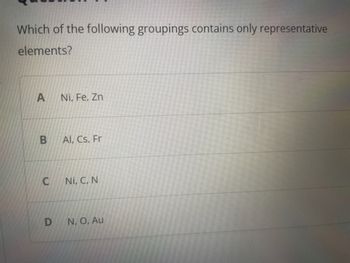
Transcribed Image Text:Which of the following groupings contains only representative
elements?
A
B
C
D
Ni, Fe, Zn
Al, Cs, Fr
Ni, C, N
N., O, Au
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the average atomic mass for an unidentified element given the following % abundance relative mass mass contribution isotope X-24 78.994 23.985041700 X-25 10.001 24.98583692 X-26 11.013 25.982592929 What is the element ?arrow_forwardWhat is the percent abundance of the rarest of three iron isotopes?arrow_forwardWhich of the following is most likely going to be a heterogeneous mixture? (A) C10H8 and H2O (B) H2S and H2O (C) O2 and O3 (D) NaCl and CH3OHarrow_forward
- Naturally occurring magnesium has the following isotopicabundances:arrow_forwardWhat is the symbol of the element in Group 4A(14) and Period 2?arrow_forwardGroups of elements in the periodic table have similar O physical properties crystal formations chemical reactivity O melting pointsarrow_forward
- Can I get help with the following question pleasearrow_forwardSilicon is the foundation of today's Microelectronics.a) Indicate which other elements from group IV of the periodic table find applications in electronics, pointing out their usefulness.b) Similarly, composite materials using elements from groups III-V are widely used. Name at least 3 of these compounds, exemplifying their respective applications.arrow_forward= ===|= | にm▼=, (13 In the compound zinc phosphide, what is the charge In the compound zinc phosphide, what is the charge on the zinc ion? on the phosphide ion? 5. Explain why a 3:2 ratio of ions is necessary for the compound zinc phosphide. 6. Is there any other ratio of zinc and phosphorus ions that could exist? For instance, could you have Zn,P or ZnP,? Explain your answer 7. Explain why you don't need to specify the number of ions in the compound you are naming ionic substances like those in Model 2. 8. Describe how the names of the nonmetal elements in Model 2 are changed when they are in their anion forms.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY