
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
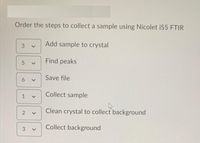
Transcribed Image Text:Order the steps to collect a sample using Nicolet iS5 FTIR
Add sample to crystal
3
Find peaks
Save file
Collect sample
1
Clean crystal to collect background
Collect background
>
>
>
>
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ALEKS - Alec Nema - Learn G c4h8o molar mass - Google Sear X + i www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZM pps Sprouts Academy:. 6 Online Tutoring C 400 Request Heade... Q Weather & Soil CH.. O CHEMICAL BONDING Identifying a molecule with one central atom from its 3D shape Check the box next to each molecule on the right that has the shape of the model molecule on the left: molecules (check all that apply) model OCOCI, O 03 O co, O BrO; 2- You c 3 4 6. O None of the above Explanation Check O 2020 O Type here to search 40 DELLarrow_forwardAnsy G A 20. Answ My C x C Write x C The X C Whe x Ten/akeCovalentActivity.do?ocator nssignnment lake 身 [ [Review Topics] [References] Use the References to access important values if needed for this question. The mass of copper(II) carbonate that is dissolved in 125 mL of a saturated solution is grams. Submit Answer Retry Entire Group No more group attempts remain Previous Next ENG 令 口 US End Insert 口し Home F7 F6 F9 F12 出arrow_forwardO Homework in Chem101 - due Su X My Questions | bartleby ( Periodic Table – Royal Society of how to take a screenshot on an + A learn.maricopa.edu/courses/1132256/modules/items/19018052 CHM151 17003 > Modules > Weeks 8 & 9 - Chapter 7 > Homework in Chem101 - due Sunday night CG 2020 FALL CRED Question 38 of Submit Account Home Complete the balanced neutralization equation for the reaction below: Announcements Dashboa |Modules HCIO:(aq) + NaOH(aq) – rd Concourse Syllabus Courses Grades O3+ D4+ Cisco Webex Groups 1 2 3 4 7 8 9. Tutoring/Learning Center Calendar Os Do Inbox (1) (g) (aq) History Na CI H. Help • Previous Next » Library 1:03 PM O Type here to search 10/18/2020 1L + 近arrow_forward
- A A DEC 11 b) Ca(OH)2 a) NaOH (s) sodium hydroxide c) KCN Til Experiment 6 Electrical Conductivity of Aqueous Solutions: Electrolytes and Nonelectrolytes d) K3PO4 (May be assigned before or after the experiment, depending on your instructor.) (Show all calculations where appropriate.) e) Na₂CO3 glish (United States) 1|1 Questions and Problems 1. Name each of the ionic compounds below and provide the equation representing its complete dissociation in water. The first one is done for you as an example. Nat (ag) + OH- (aq) f) Ba(NO3)2 ||| 8) Mg(NO₂)2 ||| EV Accessibility: Investigate tv NT Normal Focus A Bodyarrow_forwardQuestion 10 Listen If you have not weighed out enough solid compound on the balance, you should... a) pour more compound in your hand and then add it to the weigh boat on the balance. b) throw out the sample in the trash and start over. c) remove the weigh boat and add more compound, then reweigh the sample. d) throw out the sample in the waste container and start over. e) pour more compound in the weigh boat on the balance.arrow_forwardReactions: Types and E Week 18 Concept Check | Ervin x + AlpQLSdSUFh6n9jHY2beNbp9GDg0fG2L1umwbTCF2paecp714h_ihw/viewform?hr_submiss How many atoms of each type are represented in 4MG3(PO4)2? *arrow_forward
- 2. A 1.000-g sample of unknown gave 2.500 g of bis(dimethylglyoximate)nickel(II) when analyzed by reaction below. Find the weight percent of Ni in the unknown. OH Ni2+ + 2 + 2H* H.... Bis(dimethylglyoximate)nickel(II) OH DMG FM 116.12 FM 58.69 FM 288.91 4ー Z-arrow_forwardDetermine the formula weight of NH4Cl. Provide an answer to two decimal places.arrow_forwardPlease answer all question or leave for someone else to answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY