
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
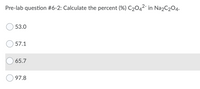
Transcribed Image Text:Pre-lab question #6-2: Calculate the percent (%) C2042- in Na2C204.
53.0
57.1
65.7
97.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please helparrow_forwardelivery/ua/la/launch/49010498/693258326/aHR0cHM6Ly9mMi5hcHAuZWRtZW50dWOuY29tL2xlYXJuZXIvc2Vjb25kYX. er Test - ICP - Chemistry 18 Select the correct answer. Give the name and formula of the compound formed when a Mn²* cation combines with a (PO4)³- anion. O A. manganese phosphate: Mn3(PO4)2 OB. magnesium phosphate: Mn,(PO4)3 OC. manganese sulfate: Mn,(PO4)3 O D. magnesium sulfate: Mn3(PO4)2 O E. manganese oxalate: Mn;(PO4)- Reset Nextarrow_forwardOk i am trying to use the info from the last question but i am still struggling with the set up.arrow_forward
- For lab 4, we combined baking soda with vinegar, according to the chemical equation NaHCO 3 + CH 3COOH ==== NaCH 3COO + CO 2 + H 2O Consider 3 experiments: (i) 2g baking soda + 5mL vinegar, (ii) 2g baking soda + 10mL vinegar and (iii) 2g baking soda + 20mL vinegar. The amount of CO 2 produced was highest for (iii) and lowest for (i). For these experiments, which of the following is true? Vinegar ran out first Baking soda ran out first They both ran out at the same time Neither ran outarrow_forwardhelparrow_forwardAnswer to #4 average % yield = 56.41% Answer to #5 average mass of CO2 produced from the unknown in the two trials = .30g CO2 For # 8 the unknown used in the experiment was unknown #2 K2CO3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY