
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please don't provide handwritten solution .....
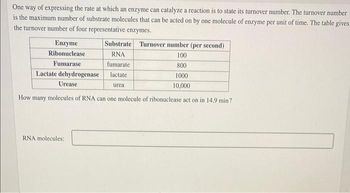
Transcribed Image Text:One way of expressing the rate at which an enzyme can catalyze a reaction is to state its turnover number. The turnover number
is the maximum number of substrate molecules that can be acted on by one molecule of enzyme per unit of time. The table gives
the turnover number of four representative enzymes.
Enzyme
Ribonuclease
Fumarase
100
800
1000
10,000
How many molecules of RNA can one molecule of ribonuclease act on in 14.9 min?
Lactate dehydrogenase
Urease
Substrate Turnover number (per second)
RNA
fumarate
lactate
RNA molecules:
urea
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemical Bonding -.. V Pennsylvania Acces... Department of Hu.. Bvlgari Man In Blac.. O CHEMICAL REACTIONS Identifying the limiting reactant in a drawing of a mixture The drawing below shows a mixture of molecules: key carbon hydrogen nitrogen sulfur oxygen chlorine Suppose the following chemical reaction can take place in this mixture: 2C,H,(g)+50,(g) → 4 CO,(g)+2H,O(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula: Explanation Checkarrow_forwardplease don't provide handwrittin solution....arrow_forwardwww-awn.aleks.com O MEASUREMENT Deducing the unit missing from the solution to a basic qu.. A student sets up and solves the following equation to solve a problem in solution stoichiometry Fill in the missing part of the student's equation. 1 kg (135D 103 1 mL 0.65 kg (0.48 L) 3 10 ? 1 Xarrow_forward
- How would someone be confident in an experimentally determined concentration? Explain the reasoning using technical terms.arrow_forwardWhat experimental technique should you use to determine the concentration of a colored solution? O measurements of volume & mass calorimetry O spectrometry O titration aarrow_forward12.1 g of goztic acid was dissolved in water weighing 87.9 G. The mass fraction (%) of acid in the solution was calculated.arrow_forward
- Can the solution be written out and solved step by step?arrow_forwardPlease don't provide handwrritten solution .....arrow_forwardMatch the following terms to their definitions: Aqueous solution, cation, centrifuge, decant, flame test, precipitate, qualitative analysis, supernate An insoluble solid formed when two solutions are mixed is called a ..... ? Group of answer choices Decant Precipitate Aqueous Solution Cation Centrifuge Flame Test Supernate Qualitative Analysisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY