
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Pls solve this question correctly instantly in 5 min i will give u 3 like for sure
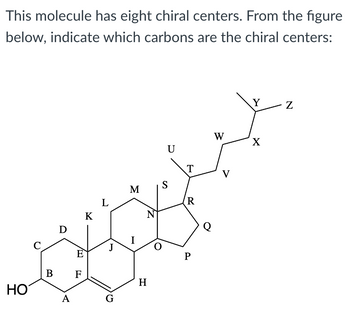
Transcribed Image Text:This molecule has eight chiral centers. From the figure
below, indicate which carbons are the chiral centers:
HO
B
D
A
K
G
M
Z
H
S
R
P
W
X
N

Transcribed Image Text:One of the stereoisomers of the molecule shown in the
previous problem is cholesterol. Including cholesterol,
how many total stereoisomers of this molecule are there?
Stereoisomers
How many enantiomers of cholesterol are there (do not
count cholesterol, and as was previously said, the
compound cholesterol is just one of the stereoisomers)?
Enantiomers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Any advicearrow_forwardI need help finding the rate law for each graph. The first graph is in order zero, the middle is in order first and the last is in order second.arrow_forwardEats PI L la To determine the stability of kinetomycin, a pharmacist dissolves an unknown amount of the drug in one litre of a saline solution. Samples are then taken at various times and sent to lab 10p allalysis. The following data are reported by the lab. Time (hr) Amount (mg) 3.5 3. Zero-order; because of a straight line on a semilog graph. 4. Zero-order; because of a straight line on a linear graph. 2. Which of the following statements is CORRECT for a ZERO-ORDER process? 1. The rate is independent of the amount or concentration of the drug. 2: The half-life is independent of the amount or concentration of the drug. 3: The rate constant is dependent on the amount or concentration of the drug. 4: The unit of rate constant is 1/time. 521 6.5 445 I. Please answer the following two multiple choice questions: 1. Please plot the above data on both linear and semilog graphs. What is the order of degradation process of kinetomycin? Why? 1. First Order; because of a straight line on a…arrow_forward
- please complete the tablearrow_forwardI-(aq) + OCI-(aq) 10-(aq) + Cl-(aq) The above reaction was studied (at a particular temperature) and the following data were obtained: [1-10 [OCI-]0 Initial Rate (mol/L) (mol/L) (mol L-1s-1) 0.360 0.100 8.42\times 10-20.045 0.100 1.05\times 10-20.090 0.200 4.21\times 10-20.180 0.200 8.42\times 10-2 Which of the following expressions for the rate law are completely consistent with the above experimental data, d[Cl-]/dt = kl OCI-][-] d[10-]/dt = k[OCI-]3 k[OCI][I] = d[OCI-]/dt-d[-]/dt = k[-]2[OC]d[1]/dt = k[-][OC] 1 pts Tries 0/5 Calculate the rate constant (k). (Units required.)arrow_forwardi shared the link so please view the video and complete the table https://www.youtube.com/watch?v=UV8KbQyF228 its just a 19 second video please checkarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY