
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
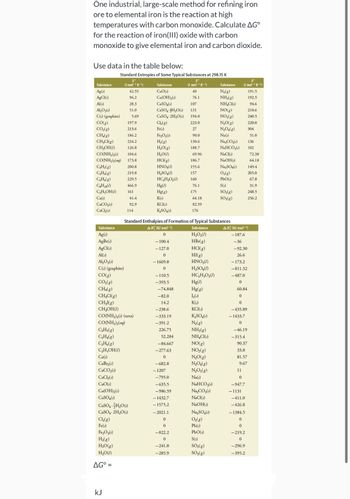
Transcribed Image Text:One industrial, large-scale method for refining iron
ore to elemental iron is the reaction at high
temperatures with carbon monoxide. Calculate AGº
for the reaction of iron(III) oxide with carbon
monoxide to give elemental iron and carbon dioxide.
Use data in the table below:
Substance
Ag(s)
AgCl(s)
Al(s)
Al₂O3(s)
C(s) (graphite)
CO(g)
CO₂(g)
CH4(g)
CH₂Cl(g)
CH₂OH(1)
C₂H₂(g)
C₂H4(g)
C₂H6(g)
C₂H18(1)
C₂H,OH()
Ca(s)
CaCO3(s)
CaCl₂(s)
197.9
213.6
186.2
234.2
126.8
CO(NH,),(s) 104.6
CO(NH,),(aq) 173.8
200.8
219.8
229.5
466.9
161
Substance
Ag(s)
AgBr(s)
AgCl(s)
Al(s)
Al₂O3(s)
C(s) (graphite)
C₂H₂(g)
C₂H₂(g)
C₂H6(g)
C₂H
Ca(s)
Standard Entropies of Some Typical Substances at 298.15 K
CaBr₂(s)
CaCO3(s)
CaCl₂(s)
CaO(s)
Fe₂O3(s)
H₂(g)
H₂O(g)
H₂O(1)
(J mol-¹ K-¹)
42.55
96.2
28.3
51.0
CO(g)
CO₂(g)
CH4(g)
CH₂Cl(g)
CH31(g)
CH₂OH(1)
CO(NH₂)2(s) (urea)
CO(NH,),(aq)
AG° =
kJ
5.69
Ca(OH)2 (s)
CaSO4(s)
CaSO4 H₂O(s)
CaSO4-2H₂O(s)
Cl₂(g)
Fe(s)
41.4
92.9
114
Substance
(J mol-¹ K-¹)
CaO(s)
40
Ca(OH)₂ (s)
76.1
CaSO4(s)
107
CaSO4+H₂O(s) 131
CaSO4-2H₂O(s)
194.0
Cl₂(g)
Fe(s)
Fe₂O3(s)
H₂(g)
H₂O(g)
H₂O(1)
HCI(g)
HNO3(1)
H₂SO4(1)
HC,H,O,(0)
Hg(1)
Hg(g)
K(s)
KCI(s)
K₂SO4(s)
-74.848
-82.0
14.2
-238.6
-333.19
-391.2
226.75
52.284
-84.667
-277.63
0
-682.8
-1207
-795.0
-635.5
-986.59
223.0
27
90.0
130.6
188.7
Standard Enthalpies of Formation of Typical Substances
AH; (kJ mol-¹)
Substance
0
H₂O₂(1)
-100.4
HBr(g)
HCI(g)
-127.0
0
HI(g)
- 1669.8
HNO3(1)
H₂SO4(1)
0
-110.5
HC,H,O,(7)
-393.5
-1432.7
- 1575.2
-2021.1
0
0
-822.2
0
-241.8
-285.9
69.96
186.7
155.6
157
160
76.1
175
64.18
82.59
176
Hg(1)
Hg(g)
1₂(s)
K(s)
KCI(s)
K₂SO4(s)
N₂(g)
NH3(g)
NH₂Cl(s)
NO(g)
NO₂(g)
N₂O(g)
N₂O4(g)
N₂O5(g)
Na(s)
NaHCO3(s)
Na₂CO3(s)
NaCl(s)
NaOH(s)
Na₂SO4(s)
0₂(g)
Pb(s)
PbO(s)
Substance
N₂(g)
NH3(g)
NH₂Cl(s)
S(s)
SO₂(g)
SO3(g)
NO(g)
NO₂(g)
N₂O(g)
N₂O4(g)
Na(s)
Na₂CO3(s)
NaHCO3(s)
NaCl(s)
NaOH(s)
Na₂SO4(s)
0₂(8)
PbO(s)
S(s)
SO₂(g)
SO3(g)
So
(J mol-1 K-1)
-92.30
26.6
AH; (kJ mol-¹)
-187.6
-36
-173.2
-811.32
-487.0
0
60.84
0
0
-435.89
-1433.7
0
-46.19
-315.4
90.37
33.8
81.57
9.67
11
0
-947.7
-1131
149.4
205.0
67.8
31.9
248.5
256.2
-411.0
-426.8
- 1384.5
0
0
191.5
192.5
94.6
210.6
240.5
220.0
304
-219.2
0
-296.9
-395.2
51.0
136
102
72.38
64.18
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the complete, balanced chemical equation for the following reaction on a separate sheet of paper. Provide products where they are not given. Assume that the reaction is spontaneous. Ammonia + oxygen → nitric acid + water Type in the coefficient and formula for the product in the equation that contains nitrogen.arrow_forwardFine particles of metallic iron can be injected underground to remediate pollution of underground aquifers by the industrial solvent trichloroethane. In one experiment, 2400L of an aqueous emulsion containing ~480 kg of Fe(0) consumed 17.0 kg of trichloroethane in 5 months. Write a balanced reaction using H2O and H+ to complete the balancing. What percentage of injected iron was used by this reaction in 5 months? Fe + C2HCl3 ---> Fe2+ + C2H4 + Cl-arrow_forwardcalculate the amount of energy required to make 235 g of aluminum at a smelting temperature of 658°Carrow_forward
- Given Zn(s) and AL(s), what observations would you make when combining AL(s) with ZnSO4(aq), and combining Zn(s) with Al(SO4)3(aq)?arrow_forwardGiven that tin is higher than copper on the activity series of metals, write the one & only possible balanced chemical equation for a redox single replacement reaction that should take place in a reaction between TWO of the following substances: Sn(s); Cu(s); Sn(NO3)2; CuCl2(aq). Also: A solid forms in the reaction. What do you think or what is the solid?arrow_forwardPlease don't provide handwritten solution ....arrow_forward
- Balance the reaction of copper plus nitrate to yield copper (II) and nitrogen dioxide. This reaction occurs in acidic solution. Cu + NO3- -------> Cu2+ + NO2 Cu(NO3)2 + 4 H+--------> Cu + 2 NO2 + 2 H2O 2 Cu2+ + 4 H2O + 2 NO2 --------> Cu + 2 NO3- + 8 H+ 10 HCl + 5 Cu + 2 NaNO3 --------> 5 CuCl2 + 2 NaNO2 + 2 H2O Cu + 4 H+ + 2 NO3- --------> Cu2+ + 2 NO2 + 2 H2Oarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward2. Lead (II) sulfide is the principle source of lead metal. To obtain the metal, the sulfide is first heated in air to produce PbO. PbS + 3/20,e - PbO + SO2) AH = -415.4 kJ (s) (8)7 The oxide is then reduced to the metal with carbon. PbO + C - Pb + CO (s) Calculate AH for the reaction of one mole of PbS with oxygen and carbon, forming lead, sulfur AH =+108,5 kJ (s) dioxide and carbon monoxide.arrow_forward
- Please don't provide handwritten solution....arrow_forwardWhen magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is oxidized and that hydrogen is reduced, write the balanced net equation for the reaction. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.) Help chemPad XXº → Greek How many electrons are transferred in the balanced equation? What quantity of useful work can be obtained when Mg is added directly to the beaker of HCI? kJ How can you harness this reaction to do useful work? This is accomplished by making ---Select--- produce a voltage. cell that ---Select--- the reduction reaction and the oxidation reaction in order to control the flow of ---Select--- through a wire toarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY