
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
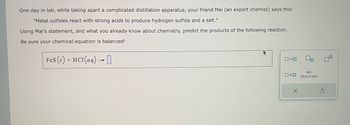
Transcribed Image Text:One day in lab, while taking apart a complicated distillation apparatus, your friend Mai (an expert chemist) says this:
"Metal sulfides react with strong acids to produce hydrogen sulfide and a salt."
Using Mai's statement, and what you already know about chemistry, predict the products of the following reaction.
Be sure your chemical equation is balanced!
FeS (s) + HCl(aq) → 0
0-0
0+0
X
NO
REACTION
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the type of the reaction, complete and balance each of the following reactions: a) b) Na(s) + CuSO4(aq) → - Single replacement. K(s) + N₂(g) → - combination. Edit View Insert Format Tools Table 12pt ✓ Paragraph BIU AT²Varrow_forwardBalance this reaction. If a coefficient is one, enter "1" explicitly. CH4+O2 → CO2 + H2Oarrow_forwardwrite the Formula Equation for the reaction between solutions of silver perchlorate and calcium bromide using the observed results for two other reactions shown below. Your answer must contain the correct subscripts and superscripts. AgClO4(aq) + NaBr(aq) -> yields precipitate CaBr2(aq) + KClO4(aq) -> no reactionarrow_forward
- Identify each of the following reactions as a synthesis, decomposition, single displacement, or double displacement reaction. 1. N(g) +3H;(g)→ 2NH,(g) 2. 2AI(s) + 3CUO(s)Al,O:(s) +3Cu(s) 3. CACO,(s)CaO(s)+CO,(g) 4. 2Li(s) + Br.(g)→ 2LİBI(s) 5. 3Sn(NO,)(aq) + 4K,PO.(aq)12KNO.(aq) +Sn:(PO)(s) 6. Mg(s) + 2HCI(aq) MgCl,(aq) + H(g) 7. Copper displaces silver in aqueous silver nitrate in a single displacement reaction as follows. Cu(s) + 2A9NO,(aq) → 2Ag(s) +Cu(NO,).(aq) a) If zinc replaces copper, will a single displacement reaction still occur? b) If a single displacement reaction occurs, predict the products of the reaction.arrow_forwarda Choose the correct balanced equation that describes this precipitation reaction: Naz CO3 (ag) + CaCl2 (ag) → CaCO3(s) + NaCl(aq) Naz CO3 (ag) + CaCl2 (ag) → CaCO3(s) + 2NaCl(ag) Naz CO3 (aq) + CaCl2 (ag) → CaCO3 (aq) + 2NACI(ag) Naz CO3 (ag) + CaCl2 (ag) → CaCO3(s) + NaCl(ag) Naz CO3 (ag) + CaCl2 (ag) → CaCO3 (ag) + NaCl(ag) b Choose the correct balanced equation that describes this precipitation reaction: Fe(C,H3O2)2 (aq) + Na, S(ag) → FeS(s) + NaC, H3 O2 (aq) Fe(C2H3 O2)2(ag) + Na2S(aq) → FeS(aq) + NaC, H3 O2 (aq) Fe(C, H3 O2)2 (ag) + Naz S(ag) → FeS(s) + NaC, H3 O2 (aq) Fe(C, H3O2)2 (aq) + Naz S(ag) → FeS(aq) + 2N2C, H3 02 (aq) Fe(C,H3O2)2 (ag) + Na2S(aq) → FeS(s) + 2NAC, H3O2 (ag) c Choose the correct balanced equation that describes this precipitation reaction: KOH(ag) + FeCl2(aq) → Fe(OH)2(s) + KCI(aq) 2КОН(ад) + FeCl, (aq) Fe(ОH)2(ag) + 2KCI(ag) 2КОН (ад) + FeCl, (aq) > Fe(ОH)2 (8) + 2КCI(aд) 2КОН (аq) + FeClz (aq) -> Fe(ОH)2 (8) + КKСІ(ад) о конад) + FeCl, (ag) - Fe(ОH)2(8) +…arrow_forward[References] Use the References to access important values if needed for this question. When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: Fe (s) + HCI (aq) →→→→ FeCl₂ (aq) + H₂ (g) 0arrow_forward
- Write the balanced NET ionic equation for the reaction when AgNO3 and KCI are mixed in aqueous solution. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. AgNO3(aq) + KCl(aq) → 1 + 04- 2 Reset 0₂ Ag 4 3 ( ) 4 K CI 5 ☐ 5 П+ 6 ☐ 6 ²+ 7 0 (s) N NR 3+ 4 8 ☐ 8 4+ 9 0 (1) (g) (aq) O •x H₂O Deletearrow_forwarda write the balanced formula equation for the reaction that occurs when the contents of the two beakers are added together. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) + Co²+ CIT Nat OH™ +arrow_forwardDesigning Chemical Reactions Lab Activity Given the list of chemicals below, write a balanced chemical equation that satisfies the reaction described. Write a description of what signs of a chemical change you would expect to see if you were able to do the reaction and write the total and net ionic equations for each reaction. Metals Bases Compound Solutions. copper (II) sulfate Ferric nitrate Acids sulfuricic acid Magnesium sodium hydroxide Indicators phenolphthalein litmus paper Zinc Calcium potassium iodide potassium nitrate Copper Iron sodium carbonate magnesium nitrate iodine solution. Single Displacement Reactions: ● Reaction #1 - Pick one reaction that works using the metal activity series: Indication of a chemical change: Total lonic Equation: Net Ionic Equation: Reaction #2 - Pick one reaction that works with water: Indication of a chemical change: Total lonic Equation: Net Ionic Equation: Reaction #3 - Pick one reaction that does not work using the halogen series: Indication…arrow_forward
- Complete and balance the equations for the given single displacement reactions. Write the reaction in molecular form. Phases are optional. If you need to clear your work and reset the equation, click the CLR button. Na(s) + H2O(l) -> Mg(s) + H2O(l) ->arrow_forwardOne way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate, solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 200. mL sample of groundwater known to be contaminated with cadmium chloride, which would react with silver nitrate solution like this: CdCl2(aq) + 2 AgNO3(aq) → 2 AgCl(s) + Cd (NO3)2(aq) The chemist adds 89.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 5.4 mg of silver chloride. Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits. E d OL Earrow_forwardUse the References to access important values if needed for this question. When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: sodium chloride (aq) + silver nitrate (aq)- silver chloride (s) + sodium nitrate (aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY