
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
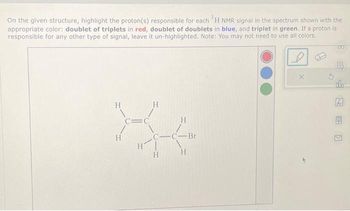
Transcribed Image Text:On the given structure, highlight the proton(s) responsible for each 'H NMR signal in the spectrum shown with the
appropriate color: doublet of triplets in red, doublet of doublets in blue, and triplet in green. If a proton is
responsible for any other type of signal, leave it un-highlighted. Note: You may not need to use all colors.
H
C=C
H
X4
H
-C-Br
H |
H
H
H
X
FOO
B
do
Ar
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of these choices best describes the interpretation of the following peak that may be recorded in a H NMR spectrum? 4.3 8 (1H, q). The underlined hydrogen atom is intended to be the one producing the peak that we are interpreting. O=C-CHCO2H O=C-CHCHX2 O=C-CHCH,X O=C-CHCH3 None of these interpretations describes this peak.arrow_forward5, Draw the correct molecule in the box to the right. Label the relevant peaks in the spectra with the proper functional group. If the absence of key peaks led to your choice, indicate which key peaks were absent.arrow_forwardWhich of these choices best describes the interpretation of the following peak that may be recorded in a 'H NMR spectrum? 3.0 8 (1H, t). The underlined hydrogen atom is intended to be the one producing the peak that we are interpreting. Ar-CHCO2H Ar-CHCHX2 Ar-CHCH,X Ar-CHCH3 None of these interpretations describes this peak.arrow_forward
- 3, Draw the correct molecule in the box to the right. Label the relevant peaks in the spectra with the proper functional group. If the absence of key peaks led to your choice, indicate which key peaks were absent.arrow_forwardAssign all of the signals in the ¹H NMR spectrum for propyl acetate. Fill in the table below with the 'H NMR data. Number each proton (or set of protons) to match the corresponding peak in the NMR. Integration Splitting Propyl acetate structure with protons labeled Signal - Chemical shift (ppm) 1 2 3 4 11 propyl acetate 10 9 8 7 6 5 ppm 2H, triplet 4 3 3H, singlet 3H, triplet 2H, sextet 2 1 Figure 3.7 ¹H NMR spectrum for propyl acetate. 0arrow_forwardThe protons indicated in the structure below would show up as a spectrum. O singlet O doublet O triplet O quartet Oseptet CH3 CH3 in a proton NMRarrow_forward
- The intensity of a signal in a ¹H NMR spectrum is determined by O Number of non-equivalent protons O Number of diasteriotiotopic carbons O The number of neighboring protons O Number of enantiotopic protons O The electronic environment of protons O Number of equivalent protons apie genann OPB88888 Witaarrow_forwardCan you elaborate step by step if this H-NMR is correct or not?arrow_forwardthe given structure, highlight the proton(s) responsible for each ¹H NMR signal in the spectrum shown with the appropriate color: singlet in , doublet in blue, and doublet of quartets in green. If a proton is responsible for any other type of signal, leave it un-highlighted. Note: You ma need to use all colors. H H C-H zy H Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY