
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Draw the structure of the compound to its matching spectrum. Mark the peaks on the spectra that helped you pick that compound.
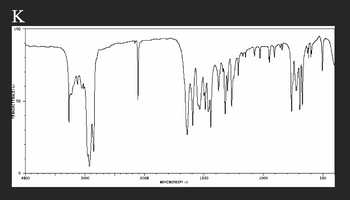
Transcribed Image Text:D
TRANSHITTANCETT
50
K
LOD
4000
3000
2000
1500
1000
500
HAVENUMBERI-II
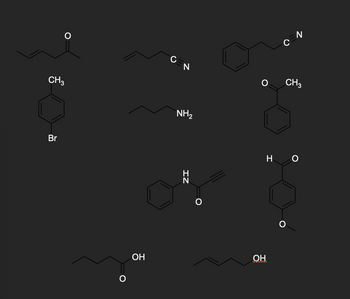
Transcribed Image Text:CH3
Br
O
0
OH
N
N
NH2
CH3
ΖΙ
HO
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- a. How many signals are in its 13C NMR spectrum?b. Which signal is at the lowest frequency? CH3CH2CH2Brarrow_forwardDraw the NMR spectra you would expect for Cl –CH2–CH2–CH2–Clarrow_forwardCounterfeit drugs are a common problem in developing regions of the world. Oftentimes, counterfeit pills are made with compounds such as lactose. A lab technician has obtained the IR spectrum shown above for a sample reported to be citalopram, an antidepressant drug. Does the IR spectrum belong to citalopram or lactose? Explain your answer by describing what feature of the IR spectrum confirms your choice and describe what feature is missing from the IR spectrum for the other compound. A. citalopram B. lactosearrow_forward
- How many unique carbons will correspond the the C NMR spectrumarrow_forwardHelp pleasearrow_forwardIdentify the following spectra. all pertinent peaks must be assigned on the spectrum (~5-6 peaks). Label the peaks on the spectrum and place the structure of the compound in the box on the lower left-hand corner of the spectrum. please on the graph circle the peakarrow_forward
- If the reference cuvette is dirty, will the spectrometer give an absorbance reading that is too high, or too low?arrow_forwardFor each pair, indicate which type of spectroscopy would most easily distinguish between the pair and give the single piece of info that would be different that supports your choice. Choices are MS, IR, UV, H NMR, C NMR.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY