
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Hello, I do not understand these questions and I am stuck. May I get help please??
Question: Draw the curved arrow mechanism for the following reaction
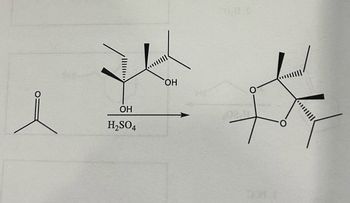
Transcribed Image Text:### Organic Chemistry Reaction Diagram
This diagram illustrates a chemical reaction commonly studied in organic chemistry involving cyclization.
#### Reactants:
- **Acetone (leftmost structure):** Represented as a simple ketone with a carbonyl group (C=O) bonded to two methyl groups.
- **Terpene-like alcohol (center structure):** A more complex structure containing hydroxyl groups (OH) on a carbon chain that exhibits stereochemistry with wedge and dash bonds indicating 3D orientation.
#### Reagent:
- **Sulfuric Acid (H₂SO₄):** A catalyst shown below the central structure, indicating its role in facilitating the reaction.
#### Reaction Process:
- An arrow points from the reactants to the product, indicating the transformation facilitated by sulfuric acid.
#### Product:
- **Cyclic Ether (rightmost structure):** A compound containing multiple rings formed through intramolecular reactions where hydroxyl groups were involved in forming ether linkages. The structure maintains stereochemistry as shown by the wedge and dash bonds.
This reaction represents a cyclization process where a linear molecule is converted into a cyclic structure with the help of an acid catalyst. Such transformations are significant in synthesizing complex natural products and pharmaceuticals.
Expert Solution
arrow_forward
Step 1: Acetal formation
Aldehyde on treatment with alcohol in the presence of acid produces acetal.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a curved arrow mechanism for the substitution reaction that will occur with the alkyl halide and nucleophile below, adding steps as necessary. Be sure to include all nonbonding electrons and all nonzero formal charges, and show all species that react or are formed in this reaction. Cl H₂Oarrow_forwardPlease help me with this i am very confused, if I have a current answer it is incorrect and I am just using this to study and I am very confusedarrow_forwardCircle the sites on diagram please. 7. Identify the nucleophilic and electrophilic sites in the reactants of the following reaction. Li-Mearrow_forward
- Follow the curved arrows and draw the product of this reaction. Ph • You do not have to consider stereochemistry.arrow_forwardfor the second picture i am supposed to do the reaction mechanism and curved arrow, which is also confusingarrow_forwardPlease step by step answer and don't use ASI Answer pleasearrow_forward
- Question 5 ? HI Edit Click on the drawing box above to activate the MarvinSketch drawing tool and then draw your answer to this question. If there is no reaction, then check the "no reaction" box below. O no reactionarrow_forwardQuestion 14 Draw the major product of the following reaction. OMe Upload Choose a Filearrow_forwardDraw the curved arrows for the mechanism. The molecule is oriented for easy arrow movement. Include dotted bonds and lone pairs in your answer.arrow_forward
- Draw the curved arrow mechanism showing how this compound would interact with ammonia, then draw the final product of this sodium ammonia reduction of an alkyne. Draw the currved arrow mechanism showing proton abstraction between the intermediate and NH3.arrow_forwardHow can we draw the structure of the unstable transition state?arrow_forward2. Draw the complete, detailed El mechanism for the following reaction (including including curved arrows). CH;OH -Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY