
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
On a sheet of paper , sketch a picture of a dehydration synthesis reaction between two amino acids. This is not meant to be tricky! Use Fig. 3.18 as a model. Be sure to identify what specific atoms on each amino acid will be involved in the reaction, and be sure and show the solid line that denotes the new covalent bond that will exist in the product. On one of your amino acids, label the amino group, the carboxyl group, and the side chain. FInally, point to and label the peptide bond in the resulting dipeptide. Take a picture of your lovely drawing and upload it here.
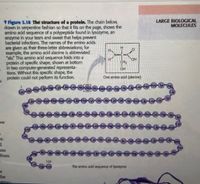
Transcribed Image Text:LARGE BIOLOGICAL
MOLECULES
V Figure 3.18 The structure of a protein. The chain below,
drawn in serpentine fashien so that it fits on the page, shows the
amino acid sequence of a polypeptide found in lysozyme, an
enzyme in your tears and sweat that helps prevent
bacterial infections. The names of the amino acids
are given as their three-letter abbreviations; for
example, the amino acid alanine is abbreviated
"ala" This amino acid sequence folds into a
protein of specific shape, shown at bottom
in two computer-generated representa-
tions. Without this specific shape, the
protein could not perform its function.
H.
H
N=C=C
HO.
CH2
OH
One amino acid (alanine)
LE Ala AlaAla
LysAng)
fhe As
Lys Ala
AsnAgAsn (The
Asn
Cys-Le-Asn-Arg-Ser
Thr-Arg
et-
al
ino
from
le
the Ala
Cys
Asn)
129
The amino acid sequence of lysozyme
Le
the
struc-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Draw a single condensation reaction occurring between two amino acids (choose any two). Clearly indicate materials entering or leaving with arrows and label all parts of one of the amino acids.arrow_forwardDescribe why G-C bonding is stronger than A-T. Why is it important?arrow_forwardThe above monosaccharides (glucose and galactose) are held together in the disaccharide lactose by: disulfide bonds ether bonds glycosidic bonds ester bonds peptide bondsarrow_forward
- Options are the same for the next slot.arrow_forward"Potassium Bromate (KBr03) is added to flour as a softener that strengthens the texture of bread. It can do this by forming disulfide bonds between two cysteine amino acids in a reaction similar to the chemical equation given below. The function of KBr03 is to remove the 2 electrons needed to form the disulfide bond as shown in the figure below. Each of the cysteine amino acids will be in a protein chain, so the reaction binds the two chains together, giving the dough cohesion. However, potassium bromate is a proven carcinogen and is only allowed in foods in the United States because bakers will add the right amount and correct It has been assumed to meet the temperature and cooking time conditions. " KBRO, + C,H¸NO,SH → KBr + H,O+C,H„N,O,S, Balance the reaction of potassium bromate (KBr03) with the cysteine molecule (C3H6 NO2 SH) by converting it into the reduced step matrix using the additive matrix method. (Kis the symbol of potassium in the question) (Homogeneous linear equation…arrow_forwardCreate an oligopeptide via dehydration synthesis using the following amino acids. O. H Tryptophan (TRP) C-C-N но сн, C-C-N. но Glycine (GLY) H H HNarrow_forward
- Which of the following best describes how the secondary structure of a protein is formed? A B с D O=U a-helix H O=C N-H R-C-H C=0 H-N H-C-R O=C N-H R-C-H C=O H-N O-C N-H R-C-H C=O H-N N-H 0= H-C-R H-N C=O R-C-4 N-H O=C H-C-R 4-1 Ç=O R-C-H N-H 0=C H-C-R (=O R-C-H B-pleated sheet ionic bonds between the R groups of the polypeptide amino acids -Uh hydrogen bonds between the carboxyl and amino groups of non-adjacent amino acids covalent bonds between the carboxyl and amino groups of adjacent amino acids hydrogen bonds between the R groups of the polypeptide amino acidsarrow_forwardHhhharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON