
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Row 2: Your answer is incorrect.
olo
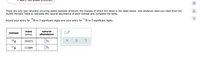
There are only two naturally-occuring stable isotopes of boron, the masses of which are listed in the table below. Use whatever data you need from the
ALEKS Periodic Table to calculate the natural abundance of each isotope and complete the table.
10.
11
Ar
Round your entry for "B to 3 significant digits and your entry for "B to 3 significant digits.
mass
natural
isotope
x10
(amu)
abundance
10B
%
10.013
11.009
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Antimony has two naturally occuring isotopes, 12 Sb and 123Sb. 12'Sb has an atomic mass of 120.9038 u, and 123Sb has an atomic mass of 122.9042 u. Antimony has an average atomic mass of 121.7601 u. What is the percent natural abundance of each isotope? 121 Sb: % 123 Sb:arrow_forwardThe element zirconium has five naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. Show your work to determine the average atomic mass. Isotope Mass % Abundance Zr-90 89.9043 51.40 Zr-91 90.9053 11.20 Zr-92 91.9046 17.10 Zr-94 93.9061 17.50 Zr-96 95.9082 2.80arrow_forwardThere are only two naturally-occuring stable isotopes of chlorine, the masses of which are listed in the table below. Use whatever data you need from the ALEKS Periodic Table to calculate the natural abundance of each isotope and complete the table. 35 Round your entry for C1 to 4 significant digits and your entry for isotope 35 Cl 37 Cl mass (amu) 34.969 36.966 natural abundance 1% % ☐x10 X 37 C1 to 4 significant digits. Śarrow_forward
- Which sample contains the greatest number of atoms? (No calculations necessary). Group of answer choices 68 g 31Ga 70 g 34Se 34 g 15P 190 g 79Auarrow_forward[Atomic mass and isotopes] An unknown element has two naturally occurring isotopes: X- 69 and X-71. The mass of X-71 is 70.9163 amu, and its natural abundance is 49.31%. Calculate the mass and natural abundance of X-69. (Hint: The atomic mass of this unknown element is 69.90 amu.) Isotope mass (amu) natural abundance (%) X-69 X-71 70.9163 49.31arrow_forwardSymbol Abundance in Nature Protons: Periodic Table Neutrons: Electrons: H. Не Li Be C Ne My Isotope Symbol Carbon-13 Abundance in Nature +1 Stable O Mass Number 13 Neutrons Atomic Mass (amu)arrow_forward
- A new element is discovered and has three stable isotopes. The atomic weight of the element is 269.7374 amu. Isotope X-268 has a measured mass of 267.9086 amu and an abundance of exactly 0.2 (infinite sig figs) Isotope X-272 has a measured mass of 271.8937 amu and an abundance of exactly 0.45 (infinite sig figs) What is the mass (in amu) of the other isotope? Report to the ten thousands place (4 decimal places)arrow_forwardfictitious element X has an average atomic mass of 122.339 u. Element X has two naturally occuring isotopes. The most bundant isotope has an isotopic mass of 121.596 u and a relative abundance of 68.36%. Calculate the isotopic mass of the leas abundant isotope. isotopic mass XTO' TOOLSarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY