
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Determining the Acid, Base, Conjugate Acid, and Conjugate Base in a Reaction Label the acid and base, and the conjugate acid and base, in the following reaction. Use curved arrow notation to show the movement of electron pairs.
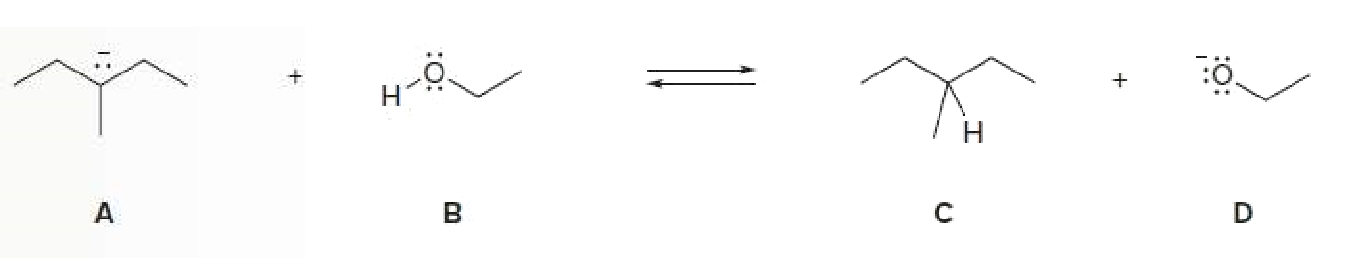
Transcribed Image Text:D
:0
:O:
Expert Solution
arrow_forward
Step 1
Please find below the curved arrow notation showing the movement of electron pairs.
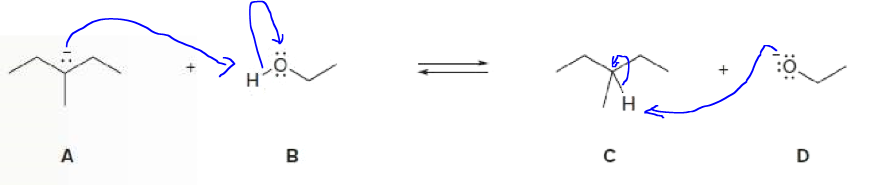
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) In class you learned that the strength of an acid or base is directly tied to the molecular structure of that acid and base. In this question you will use your knowledge of molecular structure, resonance, and electronegativity to predict the strength of a series of acids and bases. a) Write the definition of an Brønsted acids and bases. b) Arrange the acids shown below in terms increasing acid strength and assign Ka value that correspond to your ranking. Ka Values: 0.014 100 1 x 1010 sulfurous acid (H,SO3) sulfuric acid (H,SO,) fluorosulfonic acid (FSO3H)arrow_forwardThe more hydrogen ions present, the (lower/ higher) the pH, the (less/more) the acidic solution is.arrow_forwardA solution has a pH of 1. This is considered a highly acidic solution and a solution that has a pH of 14. This is considered a highly basic solution. Explainarrow_forward
- Identify the following as strong/weak/not an electrolyte. For any acids also identify them as a strong or weak acid. H3PO3 _______________ ________________ PbI2 _______________ ________________ Ba(OH)2 _______________ ________________arrow_forwardAnswer this question in under 30 minutes please. whats the difference between acids and bases.arrow_forwardAssuming the same concentration of solution and all solutions at the same temperature, when comparing different weak acids, as the pKa for the acids increases, what happens to each of the following in these acidic solutions? pH H3O+ concentration Kb of the conjugate basearrow_forward
- Which of the following choices best describes a strong acid?A) Strong acids are acids that never ionize in their solutions.B) Strong acids are acids that are only partially ionized in their solutions.C) Strong acids are acids that are nearly 85% ionized in their solutions.D) Strong acids are acids that are completely or nearly 100% ionized in their solutions.arrow_forwardIdentify acid, base, conjugate acid, conjugate base, and the relative strengths of the species involved in the reactions.arrow_forwardWhat chemical specie is the conjugate acid and which is the conjugate base in the forward direction (left to right) ?arrow_forward
- Second picture are the answer choices , and the other one is acids or bases.arrow_forwardFor conjugate acid-base pair, identify the first species as an acid or a base and the second species as its conjugate base or conjugate acid. In addition, draw Lewis structures for each species, showing all valence electrons and any formal charges. Q) H2CO3, HCO3-arrow_forwardHow many times more acidic is a solution whose pH = 1 composed to a solution whose pH =5.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY