
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
1.
What is the product for the first one and the mechanism for the second one?
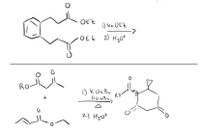
Transcribed Image Text:OEt
) Na OEt
->
OEt 2) H30t
G
RO-
1) KOŁBU
RO
HOtBu,
2) Hgut
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- alcohol. A carbocation intermediate is central to which of these reaction types? (circle) E1 E2 SN1 SN2arrow_forwardWhat does this mechanism look like? What would the final product bearrow_forward5. What halide is the strongest nucleophile in a polar aprotic solvent? Why? a. F-, Br-, Cl-, I- b. Br-, H20, TBUOK, CH3NH-arrow_forward
- Identify the nucleophile that attacks the carbocation intermediate in the acid catalyzed hydration is shown A. HO- B. H2O C. H+ D. H3O+arrow_forwardWhy does this reaction not undergo SN2 ?arrow_forwardAmong a primary, secondary, or tertiary carbocation, which is most favored for SN1 reactions? Explain whyarrow_forward
- For the following reactions, determine which mechanism they will follow, explain why the substitution of the alkyl halide doesn't affect the mechanism in an elimination reaction a. draw the mechanism showing regiochemistry of both possible products b. determine which product would be the most stable and therefore the major product c. Draw reaction coordinate diagrams and write rate laws for each reaction mechanism you Br yo you HOarrow_forwardFor the third step of the first reaction why is there a ch2-ch3 group connected to the epoxide, shouldn't there just be a ch3 group connected to it?arrow_forwardAdd the missing nucleophile to the substitution reaction in the drawing area below. Note for advanced students: You can assume the correct solvent conditions are used in this reaction. ö 80 Ararrow_forward
- what is the Nature of the leaving group (LG)? 5. what is the relative size of activation energy (Ea) for each reaction? 6. what is the Hammond's postulate? 7. what are the Relative thermodynamic stability of the reactive intermediates? 8. what is Influence of the solvent (if given) on the reactions and intermediates?arrow_forward1. Provide the mechanism of the reaction shown in the picture. 2. Explain why it cannot be done in basic conditions. 3. Why is the enol tautomer favored over the keto tautomer?arrow_forward1. Of the two unimolecular reactions shown, decide which you think would be faster, i.e., which would have a higher relative rate constant (krel)? 2. Based on your answer, draw two lines on one reaction coordinate diagram that show the relative progress of each reaction along its path to product indicated? 3. what is Bond dissociation energy of the C-LG bond? 4. what is the Nature of the leaving group (LG)? 5. what is the relative size of activation energy (Ea) for each reaction? 6. what is the Hammond's postulate? 7. what are the Relative thermodynamic stability of the reactive intermediates? 8. what is Influence of the solvent (if given) on the reactions and intermediates?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY