
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
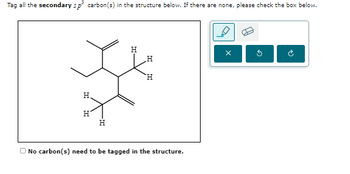
Transcribed Image Text:Tag all the secondary sp³ carbon(s) in the structure below. If there are none, please check the box below.
H
H
H
H
H
H
No carbon(s) need to be tagged in the structure.
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Please draw an alkane that has at least one primary, one secondary, and one tertiary carbon. Then take molecule and react it with F^2 (Identify all mono halogenated products for each reaction)arrow_forwardGive one possibility for the mystery reactant R in this organic reaction: R + H₂ Pu CH3 No reaction. CH3-CH₂-CH₂-CH-CH3 Specifically, in the drawing area below draw the condensed structure of what R might be. There may be more than one correct answer. Note: keep in mind that the equation above states R and H₂ are present in a 1:1 mole ratio. Click anywhere to draw the first atom of your structure. с с X cx 1)arrow_forward4. Below are the structures of some medications. Circle and identify functional groups in the structures. H₂C H₂C core MDPV Mephedrone 'N H CH3 Cocaine CH3 CH3 Methylone CH3 Aom CH3 CH3 CH3 Amphetamine NH₂ но... HO HO LaH O ACO OH 10-deacetylbaccatin IIIarrow_forward
- Hi, I need help with this question. I attempted them I just want to see if I was correct.arrow_forwardIn the name 2-chloropropane, what does the 2 represent?arrow_forward...me the family to which each organic compound belongs. The first answer has been filled in for you. compound O || CH3- C- CH₂ - CH CH₂ || CH3 I CH₂ - CH CH₂ || OH I C || O O=CIC - H I CH3 CH₂=CH-OH family aldehydearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning