
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
HELP NOW PLEASE ! URGENT!
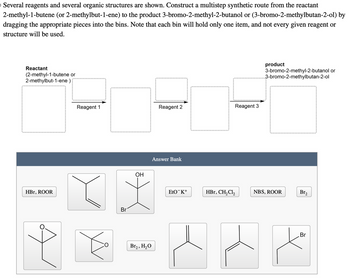
Transcribed Image Text:o Several reagents and several organic structures are shown. Construct a multistep synthetic route from the reactant
2-methyl-1-butene (or 2-methylbut-1-ene) to the product 3-bromo-2-methyl-2-butanol or (3-bromo-2-methylbutan-2-ol) by
dragging the appropriate pieces into the bins. Note that each bin will hold only one item, and not every given reagent or
structure will be used.
Reactant
(2-methyl-1-butene or
2-methylbut-1-ene):
HBr, ROOR
Reagent 1
Reagent 2
Reagent 3
Br
OH
Br₂, H₂O
Answer Bank
product
3-bromo-2-methyl-2-butanol or
3-bromo-2-methylbutan-2-ol
EtO-K+
HBr, CH2Cl2
NBS, ROOR
Br₂
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw the major organic product of the reaction. Indicate the stereochemistry via wedge‑and‑dash bonds, including explicit HH and DD atoms, at the stereogenic center. Omit byproducts such as salts or methanol.arrow_forwardWhat is the mechanism to get from the reactant to the productsarrow_forwardYour task is to convert 2-bromobutane to 1-butene in highest yield. Which reagents would you use? O KOH/H2O O KOH/CH,OH O CH3CH2ONA/CH3CH2OH O CH3ONA/CH3OH O (CH3)3COK/(CH3)3COHarrow_forward
- Please help me with the mechanism with arrows.arrow_forwardMulti-step synthesis. Give the reagents over the arrows and the intermediates between thearrows that are required to synthesize the product compound on the left from the reactant givenon the right for each retrosynthetic analysis.arrow_forwardIn the space provided below, complete each transformation by indicating all necessary reagents, and/or the major organic product(s).arrow_forward
- Please answer botharrow_forwardProvide an arrow pushing mechanism for the following transformation. Draw out a representation of the transition-state in the rate-determining step.arrow_forwardThe following reaction involves two sequential Heck reactions. Draw structural formu- las for each organopalladium intermediate formed in the sequence and show how the final product is formed. Note from the molecular formula given under each structural formula that this conversion corresponds to a loss of H and I from the starting material. Acetonitrile, CH,CN, is the solvent. 1% mol Pd(OAc), 4% mol Ph,P CH,CN C4H171 C4H16arrow_forward
- Complete the following synthesis by filling in the missing reactants, reagents, and products.• Boxes with sharp corners are structures; rounded corners are reagents (may be written or drawn).• Reagent boxes may hold multiple reagents/steps.arrow_forward. Provide the reagents necessary to carry out the following conversion. OH u NaOH/H₂O 1. NaOCH3, 2. H₂O¹ 1. (CH₁) COK, 2. BH3, 3. H₂O/NaOH/H₂O | 1.(CH3)COK, 2. HOarrow_forwardFf.144.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
