
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:O Macmillan Learning
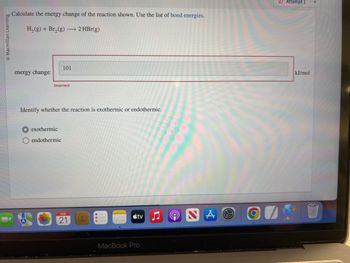
Calculate the energy change of the reaction shown. Use the list of bond energies.
H₂(g) + Br₂(g) → 2 HBr(g)
energy change:
101
Incorrect
Identify whether the reaction is exothermic or endothermic.
exothermic
endothermic
FEB
21
tv
MacBook Pro
N
A
O
Attempt 1
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reaction below, try to use method 2 to estimate the enthalpy of reaction in kJ/mol using the table of bond enthalpies above: H. -H +4023CO2 + 3H2O H H Harrow_forwardUse the graph to answer the following questions: What type of reaction is shown by the graph below? How does the bond energy of the reactants compare to the bond energy of the products? Activation energy +AH Reactants Products Time O The graph shows an exothermic The bond energy of the reactants is higher than the bond energy of the products. O The graph shows an endothermic reaction. The bond energy of the reactants is lower than the bond energy of the products. O The graph shows an endothermic The bond energy of the reactants is higher than the bond energy of the products. O The graph shows an exothermic reaction. The bond energy of the reactants is lower than the bond energy of the products. Potential energy (kJ)arrow_forwardOnly typed answer with explanation otherwise leave itarrow_forward
- Which one of the following trends appears when considering lattice energies? Anion A) The higher the charge on the ions, the higher the lattice energy. Cation F- CI- Br- |- O2- B) The more ions present in an ionic compound, the smaller the lattice energy. Lit 1036 853 807 757 2925 Na+ 923 787 747 704 2695 C) The larger the distance between ions in the ionic compound, the higher the lattice energy. K+ 821 715 682 649 2360 Mg2+ 2957 2524 2440 2327 3791 D) In general, alkali metals have larger lattice energies than alkaline earth metals with the same anions. Ca2+ 2630 2258 2176 2074 3401 Al3+ 5215 5492 5361 5217 15,916arrow_forward7) Propane is a gas commonly used to fuel gas grills and camping stoves. The structure of propane is shown below. TABLE 8.4 Average Bond Enthalpies (k)/mol) Single Bonds H C-H N-H N-N N-O N-F 413 391 O-H 463 F-F 155 C-C C-N C-O O-O O-F O-CI 348 163 146 H. H. C-F 253 C-CI 242 293 201 190 358 272 203 :-F C-CI C-Br C-I 485 N-CI N-Br 200 234 H H H 328 243 Br-F 237 S-H S-F S-CI S-Br S-S Br-CI 218 Br-Br 193 276 339 327 253 218 266 Propane 240 259 H-H 436 567 431 366 299 C-S H-F H-CI H-Br H-I Si-H Si-Si 323 226 -CI I-Br 208 175 151 Si-C Si-O 301 368 Multiple Bonds C=C C=C 614 839 615 N=N NEN N-O 418 495 941 607 SO SS 523 418 891 799 1072 a) Write the balanced chemical equation for the combustion of propane. b) Draw out lewis dot structures to represent all molecules of all products and reactants in this reaction. The number of each molecule should be reflected in the balanced equation. c) Calculate the heat of combustion for propane using the bond enthalpies in the table. d) 1.00 mol of…arrow_forwardDetermine whether each item is describing an endothermic or exothermic reaction by checking the appropriate circle : Forming new bonds Breaking bonds Boiling is Endothermic Exothermic Condensing is О О Energy is released О Energy is absorbed 6 poinarrow_forward
- QUESTION 6 Which compound should have the lowest lattice energy? RbCl Rbl RbF RbBr Click Save and Submit to save and submit. Click Save All Answers to save all ar 854 NOV 17 atv Sarrow_forwardthe correct resonance structure are ( a&B, a&c, a&d, b&c, b&d, or c&d). The most important contributer is (H, A, B, C, D) and second most important is (H, A, B, C, D)arrow_forwardstion 10 of 12 > zinc Zn(s) aluminum Al(s) 2 K(s) + S(s) -> Determine whether each reactant gains or loses electrons to form an ionic compound. Fe(s) + L₂ (s) K₂ S(s) - Fel₂ (s) Resources Answer Bank loses e xove op! gains e Hilltarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY