
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
is this correct?
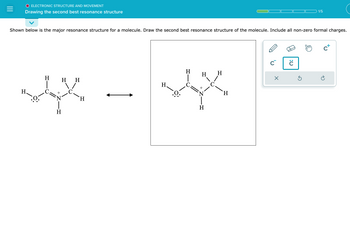
Transcribed Image Text:**Electronic Structure and Movement**
**Drawing the Second Best Resonance Structure**
---
**Illustration Description:**
Two resonance structures of a molecule are displayed side by side with a double-headed arrow between them, indicating resonance. The molecule consists of a chain with the following elements and bonds:
- A nitrogen atom (N) with a positive charge, bonded to three hydrogen atoms (H).
- A carbon atom (C) double-bonded to an H and a nitrogen atom.
- Another carbon atom single-bonded to the first carbon atom and two hydrogen atoms.
- An oxygen atom (O) bonded to the first carbon atom, with three lone pairs of electrons and a single bond to a hydrogen atom.
**Instructions:**
Below the diagram, it reads: "Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges."
The current resonance structure is shown on the left, and a box on the right prompts the user to draw the second best resonance structure. Several editing tools are visible, including tools to draw bonds, change charges, and add lone pairs of electrons.
---
**Educational Context:**
In this exercise, students are tasked with drawing the alternative resonance structure of a given molecule while considering the formal charges and electron movement. This enhances understanding of molecular stability and electron delocalization.
Expert Solution
arrow_forward
Step 1
Resonance structure :- The one structure of molecule in which the π-electrons and lone pair of electrons delocalise to form another possibile structure of that molecule is known as resonance structure.
In resonance structure all atoms position are localised only π-electrons and loan pair of electrons delocalise.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the coordinate bond in the compound and identify which atom was the electron acceptor for that bond. O 1 and N 5 and B O2 and C O4 and N 1 and B O2 and N O3 and C 5 and N marrow_forward3D16283.34693 16.3 mapbe -> 2. The volume of a cylinder is calculated as V= r?h where nr2 is the area of the circle. If a gallon of kerosene were spilled on a still lake and the height of the slick is, 1.386 nm, what would be the area in m? of the oil slick on the surface of the pond? 1 gal = 3.8 L; 1 nm = 1 x 10-9 m; 0.81 kg = 1 L; 1 kg = 2.206 Ibs; %3D %3D %3D %3D 1000 cm3 = 1.0 liter %3D 1.386nm |1X10-m Inm Aluminum has a density of 2.70 g/cm3. What would the volume be in in3 of a piece of alarrow_forwardThis is not correct and neither is AG.arrow_forward
- Determine the slope of the line. 3. 2+ 1+ 1 4 6. -1+ X-axis -2. -34 -4+ O -2 O 2 0.5 O 0.5 N. y-axis -1arrow_forwardO Course Home b Search results for 'use dimension x -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=b10f10fff5c853184f7070f2a1f9d5b#10001 : Apps YouTube о Марs E Connect - To Do As... оссс Моodle P chem work b help I Balance Chemical E. Gmail II Review | Constants | Periodic Table Scores Use the kinetic molecular theory of gases to explain each of the following. eТext Document Sharing Part A User Settings Gases move faster at higher temperatures. Course Tools > O at a higher temperature, the number and force of the collisions against the walls of the container is greater. O the attractive forces between the particles of a gas increase with temperature. According to kinetic molecular theory, at a higher temperature, gas particles have greater kinetic energy. as temperature increases, the concentration of gas molecules in a container increases. Submit Request Answer Part B Gases can be compressed much more than liquids or solids. According to kinetic molecular…arrow_forwardQuestion: provide the name of this moleculearrow_forward
- AutoSave w | homework – Saved to my Mac OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe Paste BIU V ab x, x A v I v A v No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity Please answer the following questions fully and to the best of your ability 1) Please state how you would synthesize the following polymer below and show the mechanism behind the reaction. Note that this also means you need to tell me which molecule you are starting with as well as specifying all conditions required. он OH 2) Will either the polymer or the monomers you used to make it show peaks if IR spectroscopy analysis was done on them, and if so where? How about if UV-vis spectroscopy was performed instead? Page 1 of 1 93 words E English (United States) O Focus 白arrow_forwardWhich is not a type of molecular motion? Rotation Translation O Vibration O Shimmyarrow_forwardThe heat of formation at 25°C of liquid acetone, CH,C(0)CH,(1) is -247.5 kJ/mol. Which of the following is the correct heat of formation reaction for CH,C(O)CH,() )?arrow_forward
- The room temperature phase of lithium is a liquid. True O False QuickNavarrow_forwardn + Сизонarrow_forwardA www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IJgXZp57itWHhRgilODc5Mqv... G Apps CSU Study/Teach Abro... GACE Testing O Degree Related E E-Tandem E Grade Calculator 国Re Korean O STATES OF MATTER Identifying the intermolecular forces between atoms, ions and... Kyli. What kind of intermolecular forces act between a bromine (Br,) molecule and a tetrachloroethylene (C,Cl4) molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY