
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
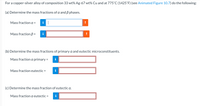
Transcribed Image Text:For a copper-silver alloy of composition 33 wt% Ag-67 wt% Cu and at 775°C (1425°F) (see Animated Figure 10.7) do the following:
(a) Determine the mass fractions of a and B phases.
Mass fraction a =
Mass fraction ß =
(b) Determine the mass fractions of primary a and eutectic microconstituents.
Mass fraction a primary =
Mass fraction eutectic =
(c) Determine the mass fraction of eutectic a.
Mass fraction a eutectic =
i
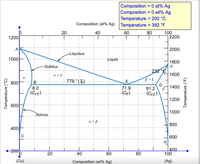
Transcribed Image Text:Composition = 0 at% Ag
Composition = 0 wt% Ag
Temperature = 200 °C
Composition (at% Ag) Temperature = 392 °F
60
T
20
80
40
100
2200
1200
A
2000
Liquidus
1000
Liquid
1800
-Solidus
232°C
a + L
1600
a
800
779 °(TE)
B+L
G
91.2
1400
8.0
(Cuɛ)
71.9
(CE)
(CBE)
1200
600
1000
Solvus
a + B
800
400
600
200
400
100
20
40
60
80
(Cu)
Composition (wt% Ag)
(Ag)
Temperature (°C)
Temperature (°F)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Doard.learn.xythos.prod/579872cf81db6/2987817?X-Blackboard-53-Bucket-blackboard.learn.xythos.prod&X-Blackboard-Expiration= S 1/2 CHEM1407 HF HBr #1) Identify if the following substances are a weak acid, strong acid or neither and write the dissociation equation for the acids LiOH H₂SO3 NH3 HC₂H302 HCIO4 HCIO HF H₂SO4 #2) Identify the following as a monoprotic, diprotic, or triprotic acids H3PO4 HC1O2 HNO3 100% + H₂C₂O4 Homework Ch 16, Sec 6A H Name: 63°F Mostly cloudyarrow_forwardQ5/ A gaseous mixture of CO, CO2, CH4, and N2 is analyzed with a gas chromatograph. The output appears on a computer monitor, as shown in below table. Component co CH. No. moles 40 25 м 28 16 CO2 80 44 CH 20 N2 28 For each of the three species, the area under the peak is approximately proportional to the number of moles of the indicated substance in the sample. From other information, it is known that the molar ratio of methane (CH.) to nitrogen is 0.200. (a) What are the mole fractions of the four species in the gas? (b) What is the average molecular weight of the gas?arrow_forwardA bedroom has a volume of 108 m^3. What is it volume in cubic kilometers (Km^3)?arrow_forward
- Please ONLY do #4 not #2arrow_forward02A: 2.1-2.5 and Accuracy Handout (70 min) ulate Figures of Merit for Trueness ▾ Part A. Absolute Error A breathalyzer is a portable electrochemical instrument that measures the concentration of ethyl alcohol in a sample of exhaled breath A forensic chemist checks the calibration of a breathalyzer by testing a standard air sample known to contain 0.174 mg/L of ethanol seven times. The average value of these seven replicate measurements is found to be 0 157 mg/1 Calculate the absolute error of the breathalyzer. 1971 ΑΣΦΑ Submit Previous Answers Request Answer ? Previous x Incorrect; Try Again: 4 attempts remaining Q Search mgl ly Colle 16 of 17 E dx Next 11:44 PM 1/31/202 BANG &arrow_forwardme x C Thermo X Cosmet x C Laptops x M Inbox (7 X C Buy TCL X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content T K Saturat X ZA a Amazon x C Show Ye X G what's a X Email- 1 X ☆ b The dia x h EX E ct the rato at which + 4. Consider the interconversion of A and B. Suppose in the absence of an enzyme, the forward rate constant KF is 104 s¹ and the reverse rate constant KR is 106 S¹. Calculate the equilibrium constant K. How would the presence of an enzyme affect the value of K? Karrow_forward
- only answer question four with the correct answer please.arrow_forwardie/litrs) Temperalive/Veding 120 aso 240 Bl60 ons L000 125. 12)Qut in the Gruph 1beku the dua abue Chaler's Law SO0 400 200 00 025 O50 Volamliter0 1125. 1is0arrow_forwardPerform the following calculations to the correct number of significant figures. [(2.33 x 10^6) / 42.370] + 132.99arrow_forward
- ← Chrome File Edit View History Bookmarks Profiles Tab Evolution of the Gex tab → 13.1 Solution Form.... M Gmail YouTube Maps Ghess law organic c..... M esc caps lock 75% D Sat 10:20 PM a Gp and reactants-G X Significant Figures X Bb Department of Che X M Mathway | Algebra x A ALEKS-Lara Althax www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-lvTqeviKFP6W0cqJcWJdIACROQwyw24GWHin4XFyQvsTuo6422Ls2TWa4xMOJZaDSVCHQol7p3... ☆ O KINETICS AND EQUILIBRIUM Using Le Chatelier's Principle to predict the result of changing... Carbon disulfide and oxygen react to form carbon dioxide and sulfur dioxide, like this: CS₂(g)+30₂(g) → CO₂(g) +2SO₂(g) perturbation Some O₂ is removed. Some CO₂ is added. Suppose a mixture of CS₂, O₂, CO₂ and SO₂ has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. Explanation 1…arrow_forwardSTARTING AMOUNT esc Tap here or pull up for additional resources ! 1 Q A X * 2 30² F2 W S # 3 80 F3 E D $ 4 (1000)¹ 0.01 100 Q F4 R ADD FACTOR * ( ) FL % 5 J F5 T m³ Convert 7.6 cm³ to m³ (0.1)* (0.01) 1000 (100)³ 7.6 x 10-4 G Question 25 of 31 ^ 6 cm³ F6 Y - ANSWER 0.076 m & 7 H (1)³ 7.6 cm K F7 * 00 0.1 7.6 x 10⁰ 8 RESET J 12 DII FB 1 1 ( 9 K F9 ) O O 4 F10 L Parrow_forward2 Automobile air bags inflate following a serious impact. The impact triggers the following chemical reaction. 2NaN3 (8)→ 2 Na(s) + 3N₂ (9) S W stry.com/myct/itemView?assignmentProblemID=213377858&attemptNo=2&offset=next X 7 # 3 E D 80 C $ 4 R 888 F V % 5 FS T Y G A Part A 6 B If an automobile air bag has a volume of 11.2 L, what mass of NaN3 (in g) is required to fully inflate the air bag upon impact? Assume STP conditions. IVE ΑΣΦ Provide Feedback m = Submit MacBook Air F6 Y H & 7 Request Answer N 40 U J * 8 @ C PwC FB I M ( 9 K DD ? O ) O g L command 3 F10 P . : ; Review I Constants I Periodic Table I { + [ option = 11 ? 1 I 323) Next > 1 deletearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY