
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
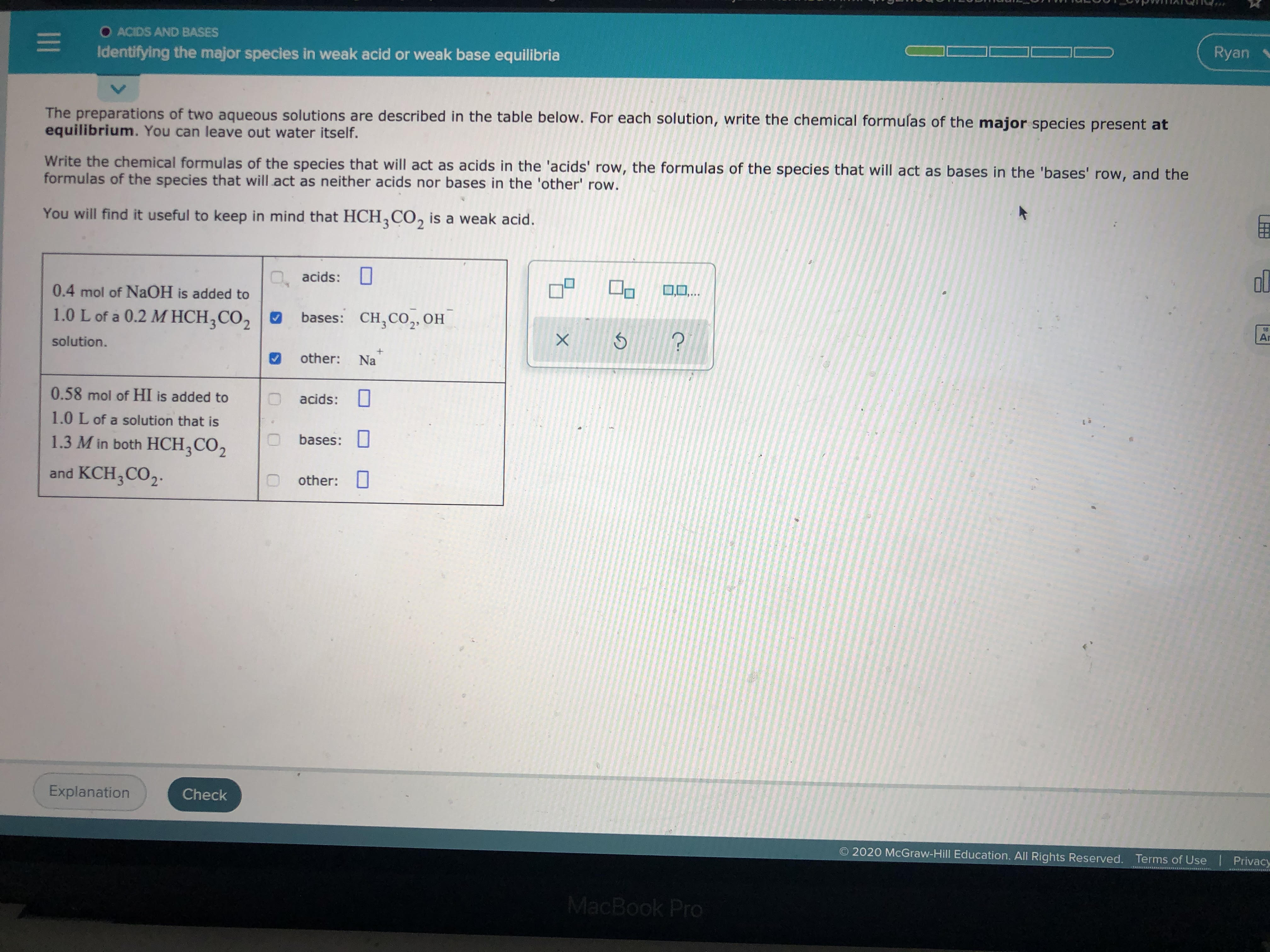
Transcribed Image Text:O ACIDS AND BASES
Identifying the major species in weak acid or weak base equilibria
Ryan
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HCH,CO, is a weak acid.
O, acids: 0
ol
0.4 mol of NAOH is added to
0,0,..
1.0 L of a 0.2 M HCH;CO2 O
bases: CH,CO,OH
18
Ar
solution.
other:
Na
0.58 mol of HI is added to
acids: I
1.0 L of a solution that is
1.3 M in both HCH;CO2
bases:
and KCH;CO,.
other:
Explanation
Check
2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy
MacBook Pro
1II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- Write equations to illustrate the acid-base reaction when each of the following pairs of Brnsted acids and bases are combined: Acid Base a.HOCl H2O b.HClO4 NH3 c.H2O NH2 d.H2O OCl e.HC2O4 H2Oarrow_forwardExplain why equilibrium arrows are used in the ionization equations for some acids.arrow_forwardWrite a formula for the conjugate base formed when each of the following behaves as a Brnsted acid: a. HSO3 b. HPO42 c. HClO3 d. CH3NH3+ e. H2C2O4arrow_forward
- Aqueous Solutions of Acids, Bases, and Salts a For each of the following salts, write the reaction that occurs when it dissociates in water: NaCl(s), NaCN(s), KClO2(s), NH4NO3(s), KBr(aq), and NaF(s). b Consider each of the reactions that you wrote above, and identify the aqueous ions that could be proton donors (acids) or proton acceptors (bases). Briefly explain how you decided which ions to choose. c For each of the acids and bases that you identified in pan b, write the chemical reaction it can undergo in aqueous solution (its reaction with water). d Are there any reactions that you have written above that you anticipate will occur to such an extent that the pH of the solution will be affected? As pan of your answer, be sure to explain how you decided. e Assume that in each case above, 0.01 mol of the salt was dissolved in enough water at 25C to make 1.0 L of solution. In each case what additional information would you need in order to calculate the pH? If there are cases where no additional information is required, be sure to state that as well. f Say you take 0.01 mol of NH4CN and dissolve it in enough water at 25C to make 1.0 L of solution. Using chemical reactions and words, explain how you would go about determining what effect this salt will have on the pH of the solution. Be sure to list any additional information you would need to arrive at an answer.arrow_forwardWhen someone hyperventilates, a condition known as respiratory alkalosis can occur. Explain the cause and effect of respiratory alkalosis. Hint: Reference Exercises 146 and 147.arrow_forwardFor oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen atom? b. the electronegativity of the element bonded to the oxygen atom that bears the acidic hydrogen? c. the number of oxygen atoms? How does the strength of a conjugate base depend on these factors? What type of solution forms when a nonmetal oxide dissolves in water? Give an example of such an oxide. What type of solution forms when a metal oxide dissolves in water? Give an example of such an oxide.arrow_forward
- Write the acid ionization constant expression for the ionization of each of the following monoprotic acids. a. HF (hydrofluoric acid) b. HC2H3O2 (acetic acid)arrow_forwardHow do the components of a conjugate acid—base pair differ from one another4? Give an example of a conjugate acid—base pair to illustrate your answer.arrow_forwardEach box represents an acid solution at equilibrium. Squares represent H+ ions, and circles represent the anion. Water molecules are not shown. Which figure represents a strong acid? Which figure is a weak acid?arrow_forward
- Classify each of the following statements as true or false: aAll Brnsted-Lowry acids are Arrhenius acids. bAll Arrhenius bases are Brnsted-Lowry bases, but not all Brnsted-Lowry bases are Arrhenius bases. c HCO3 is capable of being amphoteric. d HS is the conjugate base of S2. eIf the species on the right side of an ionization equilibrium are present in greater abundance than those on the left, the equilibrium is favored in the forward direction. f NH4+ cannot act as a Lewis base. gWeak bases have a weak attraction for protons. hThe stronger acid and the stronger base are always on the same side of a proton transfer reaction equation. iA proton transfer reaction is always favored in the direction that yields the stronger acid. jA solution with pH=9 is more acidic than one with pH=4. kA solution with pH=3 is twice as acidic as one with pH=6. lA pOH of 4.65 expresses the hydroxide ion concentration of a solution in three significant figures.arrow_forwardCalculate the concentration of all solute species in each of the following solutions of acids or bases. Assume that the ionization of water can be neglected, and show that the change in the initial concentrations can be neglected, Ionization constants can be found in Appendix H and Appendix I. (a) 0.0092 M HCIO, a weak acid. (b) 0.0784 M C6H5NH2, a weak base. (c) 0.0810 M HCN, a weak acid. (d) 0.11 M (CH3)3N, a weak base. (e) 0.120 M Fe(H2O)62+ a weak acid, Ka=1.6107arrow_forwardWhen perchloric acid ionizes, it makes the perchlorate ion, ClO4. Draw the Lewis electron dot symbol for the perchlorate ion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
