
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
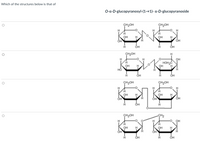
Transcribed Image Text:Which of the structures below is that of
О-а-D-glucopyranosy-(1->1)- а-D-glucoругanoside
CH2OH
CH2OH
O. H
H.
он
H.
он
H
OH
ÓH
CH2OH
HOH2
H.
CH2OH
CH2OH
O. H
H.
он
он
H
OH
он
CH2OH
H.
о, он
он
H
он
он
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- sucrose + acid ------->arrow_forwardIdentify the structure of O-a-D-sorbopyranosyl-(24)-B-D-glucopyranose below. The following structures will help: НО Н ОН х Н H H D-sorbose Н OH CH₂OH Н Н Н OH HO Н H HO H н HO ОН Н н Н О ОН O H Н CH₂OH OH OH н 0 CH2OH Н OH Н H OH Н CH₂OH H ОН Н OH Н Н Н РО OH Н HO OH н н -O CH2O Н O H H OH н Н OH CH₂OH о CHOH H H Н Ко OH H OH Н Н OH Н OH Н OH Н CH₂OH -OCHOH H OH O CH OH Н OH Н Н CH₂OH ОН он OH OH H OH H O OH CH₂OH OH ОН Н O OH OH D-glucose Н ОН Н ОН ОНarrow_forwardCellulose and glycogen are both polymers of glucose, but they have very different functions. Select all of the statements below that are true (this is a multi-select question). One important difference between cellulose and glycogen is that the cellulose has a(1→6) branches, which greatly increases the "connectiveness" within the structure. One important difference between cellulose and glycogen is that cellulose is a B(14) linked glucan, while glycogen is an a(1→4) linked glucan. The major cause of the functional difference is that glycogen is stored in the cytosol, whereas cellulose is a component of the cell walls. Cellulose is flexible due to the noncovalent interactions between the B(1-4) linked strands of glucose: the polymer can bend without breaking covalent bonds. Cellulose is more "stretchy" than glycogen, since its structure is held together only by the relatively weak hydrogen bonds.arrow_forward
- What is the structural difference between glucose and (a) β-D-glucuronate,(b) β-D-glucosamine, (c) N-acetyl-β-D-glucosamine?arrow_forwardWhich of the two structures, (Betaxolol) or (Sotalol) would be more contraindicated to treat a patient with hypertension and a history of depression, sleep disorders or psychosis? cr -o- CH2-CH-CH2-NH2-CH(CH3)2 -H2C-O-H2C-H2C- Он (Betaxolol) CH- -CH2-NH2-CH(CH3)2 OH Cr H3C-S-HN- (Sotalol)arrow_forwardIs this an L or a D amino acid? Explain.arrow_forward
- Saccharide Y is a tetrasaccharide. To determine its linkages, glycopeptide X was exhaustively methylated, effectively methylating saccharide Y. Cleavage gave a polypeptide and the following products: 2,3,4-tri-O-methyl-a-D-glucopyranoside acid, 2,3- di-O-methyl-ß-D-glucopyranoside, 1,3,4-tri-O-methyl-ß-D-glucuronic acid and 1,4,6-tri-O- methyl-ß-D-N-acetylgalactosamine. Further treatments to the isolated tetrasaccharide gave the following results: A. Treatment of saccharide Y with an a-1,4 glycosidase gave a trisaccharide and glucopyranose B. Treatment of saccharide Y with a 2,6-glycosidase also gave a trisaccharide and glucuronic acid. Draw the structure of the saccharide.arrow_forwardHow is the glycerol 3-phosphate required for phosphatidate synthesis generated?arrow_forwardNote:- Provide detailed explanation for the reaction that involves the removal of nitrogen from an alpha-amino acid, forming an alpha-keto acid. Please explain with the help of an example.arrow_forward
- Complete the following table by providing the Fischer and Haworth Projections of the given sugars. (Please be careful with the alpha and beta, and the complete and abbreviated) (not a graded question) Sugar Fischer Projection Haworth Projection (Complete) alpha-anomer Haworth Projection (Abbreviated) beta-anomer L-Galactose L-Fructosearrow_forwardWhat is the 1-letter code for the amino acid glutamine? N E Q Garrow_forwardWhich structure is the correct Haworth representation of a-D-glucopyranosyl-(1-3)-B-D-galactopyranose? он OH но- OH OH OH OH он он он но- но HO OH OH он он OH OH Он OH он OH OÀ Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON