
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please answer 4th part only
Both the photos are same
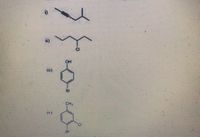
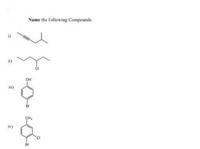
Transcribed Image Text:Name the following Compounde
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION Bromine has two isotopes, 79Br and 81Br. To find out the isotopic abundance of each bromine isotope, a sample of Br, was injected to a mass spectrometer which subsequently released the spectra shown below. From each spectrum height was measured and tabulated. Identify each peak in terms of formula. For example, the first peak is identified as 79Br. DATA Mass Number Peak height, cm 79 4.12 81 4.12 158 3.34 160 6.68 162 3.34 79 81 158 160 162 MASS, amu Relative Abundancearrow_forwardModule 2 Science Journal Lesson 1 Practice 1- Atoms: The Space Between Video | 1. What are some characteristics of the "space" inside an atom? 2. If an atom is mostly empty space, what keeps other matter from moving through the space inside an atom?arrow_forwardPractice Task/Assessment Complete the table below. ION TYPE OF ELEMENT TYPE OF ION CHARGE 1. Zn+2 2. Cr+3 3. At- Fill in the blanks. For the ionic compound Ag2S, there is/are ___ Ag+ ions and __ S- The total charge of Ag2S is ___. ___ cation and ___ anion formed Potassium iodide (KI). ___ cation and ___ anion formed Potassium chloride (KCl). Write the chemical formula of the ionic compounds formed from the following ions. Potassium and Iodine ions Mg +2 + Br-1arrow_forward
- Use the References to access important values if needed for this question, An ion from a given element has 20 protons and 18 electrons. What is the charge on the ion? What is the name of the element? What is the symbol for the ion? Submit Answer Try Another Version 2 item attempts remaining ot pt ptarrow_forwardPlease help me with chemistry practicearrow_forwardName ionic (a) What is the name of the compound with the formula Al2(CrO4)3? (b) What is the the formula for silver nitrite? Check & Submit Answer Show Approacharrow_forward
- Which of the steps described here prevents the contamination of just-washed hands? use of a paper towel to turn off a faucet O lathering under running water for 30 seconds wetting the hands before applying soap use of hot water.arrow_forward1 pt 1 pt pt pt pt pt pt ot of ot ot ot ot t ot Use What is the name of the alkali metal that is in period 5? What is the name of the halogen that is in period 5? What is the name of the alkaline earth metal that is in period 6? What is the name of the noble gas that is in period 1? Submit Answer Try Another Version 1 item attempt remaining Visited It neearrow_forwardLearning Goal: Atoms with the same number of protons but different numbers of neutrons are called isotopes. Some elements only have one naturally occurring isotope, while others such as carbon have two or more. In a naturally occurring sample, isotopes of each element are present in a certain percentage amount called the percent natural abundance. The mass number of an isotope is the sum of the number of protons and the number of neutrons for a particular isotope and is symbolized by A. A = number of protons + number of neutrons There are several different types of isotope notation. For example, a carbon isotope that has an atomic number of 6 and a mass number of 13 could be symbolized in any of the following ways: 13C, carbon-13, or C-13. b lock Part A Enter the appropriate symbol for an isotope of potassium-39 corresponding to the isotope notation X. Express your answer as a chemical symbol using isotope notation. ►View Avallable Hint(s) esc control Submit Part B Complete previous…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY