
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
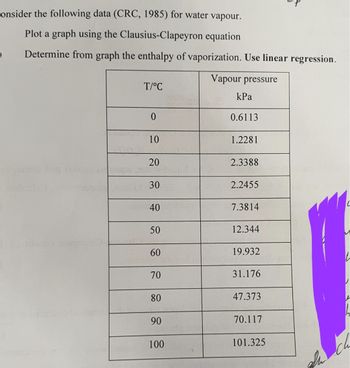
Transcribed Image Text:onsider the following data (CRC, 1985) for water vapour.
Plot a graph using the Clausius-Clapeyron equation
Determine from graph the enthalpy of vaporization. Use linear regression.
Vapour pressure
bus colo
T/°C
0
10
20
30
40
50
60
70
80
90
100
kPa
0.6113
1.2281
2.3388
2.2455
7.3814
12.344
19.932
31.176
47.373
70.117
101.325
l
Expert Solution
arrow_forward
Step 1
Here we are required to find the Enthalpy of vaporization.
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the shape of the binary liquid -vapour phase diagram for acetone and chloroform?Does it show a positive or negative deviation from Raoult's lawarrow_forwardSolid-liquid phase diagram of naphthalene-biphenyl system was formed by determination of arrest and break points in cooling curves of different composition mixtures as follows 82 283 2222 90 80 Temperature (°C) 111 70 1 50 40 30 20 10 0 Area Area 1 0.2 Phase Diagram Area Il Area IV Area III 0.8 0.4 0.6 Mole fraction of naphthalene 1 a. Write the phases of naphthalene and biphenyl in different areas on graph (assume that these molecules are completely miscible in liquid phase and immiscible in solid phase) of phases Explanations (Which phases present?) 12 b. Explain briefly the eutectic point and explain its position on grapharrow_forwardIn this lab, you will start off by calculating the number of moles of air molecules inside a flask containing 20 mL of water by cooling the flask down to near 0°C and assuming the vapour pressure of water is negligible at that temperature. If the volume of the air inside the flask is 145.2 mL, the gas pressure sensors reads 101.3 kPa and the temperature sensor reads 3.7 °C, how many moles of air are present in the flask? Express your answer in mol. Assume R = 8.314 J mol-¹ K-¹. Answer:arrow_forward
- 1- what if B = benzene, A = methanol, what are the dominant interactions between A and B, B and B and A and A and what is their dependence on the distance of the molecules? What can you follow about the strength of these interactions from the phase diagram? 2- Indicate in the figure which limiting cases you can identify. Name the limiting cases and write down the equations for it. Explain in which situations they hold. 3- You set up a solution of 70 mole % A and 30 mole % B. Calculate from the diagram of A in the gas phase. What does this indicate for a possible separation of A and B and how could you get that?arrow_forward140 120 Super- critical fluid A 100 CO,(s) CO,(1) 80 Critical point 30.98°C, 60 72.79 atm 40 B 20 CO2(g) Triple point – 56.57°C, 5.11 atm -80 -60 -40 -20 20 40 Temperature (C) What phase of carbon dioxide has the lowest chemical potential at 100 bar and 320 K? O cole) supercritical CO, O all CO, phases have the same chemical potential O co2/s) Pressure (atm)arrow_forwardAcetone has a vapour pressure of 53.3 kPa at 39.5 °C and 202.6 kPa at 78.6 °C. Estimate the acetone's critical pressure by knowing that Te is 234.95 °C. 1414 kPa 2414 kPa 5414 kPa 6414 kPaarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY