
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The intermediate shown here is formed during the hydroxide-ion-promoted hydrolysis of the ester group. Propose a mechanism for the reaction.
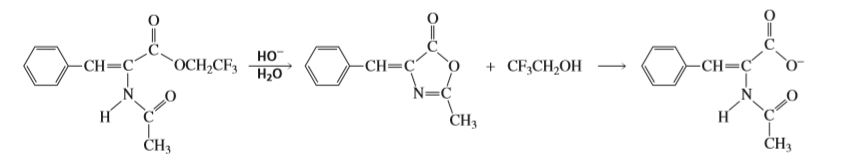
Transcribed Image Text:но
OCH2CF3
-CH=C
-CH=C
+ CF;CH,OH
-CH=C
Н,о
N=C
CH3
H'
ČH3
ČH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide a mechanism that accounts for the outcome of the following hydrolysis reaction. H3O+ ?arrow_forwardConsider the following reactants: Br Would elimination take place at a significant rate between these reactants? If you said elimination would take place, draw the major products in the upper drawing area. If you said elimination would take place, also draw the complete mechanism for one of the major products in the lower drawing area. If there is more than one major product, you may draw the mechanism that leads to any of them. Major Products: Oyes поarrow_forwardLearning Complete the electron-pushing mechanism for the base-promoted hydrolysis of an ester shown in the panels. Add atoms, bonds, nonbonding electron pairs (lone pairs), charges, and curved arrows. Step 1: Complete the structure and add curved arrows. Step 2: Complete the structure and add curved arrows. ot HAarrow_forward
- H3C H3C H3C OH Amides can be hydrolyzed to yield carboxylic acids. The conditions are much more severe than those for other carboxylic acid derivatives. Acidic hydrolysis occurs by addition of water to the protonated carbonyl, followed by proton transfer to make the nitrogen a better leaving group. The reactions are reversible, and the reaction is driven to completion by protonation of NH3 at the end. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions NOC XT .H + NH3 NH₂ -OH H3O+ H₂O: NH3 H3C :0: OH NH3 H3O+arrow_forwardNonearrow_forwardPerform the following synthesis. The number of steps in my path is provided. 5 steps OH SHarrow_forward
- Label the reactants with either electrophile, nucleophile, base or acid.arrow_forwardDraw the mechanism for the reactions between the given carboxylic acid and -OH in H2Oarrow_forwardDraw the entire extended reaction mechanism (curly arrows) for this reaction но- OH HO HO OMe H OMe OMe HO HO OMearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY