
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Show the mechanism for the all the steps involved the following reaction.
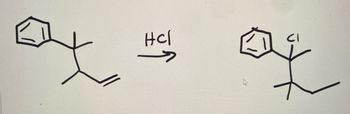
Transcribed Image Text:맛
Hel
디
몇
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What elementary step is represent by the curved arrows shown below Nucleophilic Elimination Bimolecular Nucleophilic Substitution Coordination Proton Transfer Electrophilic Elimination Electrophilic Addition Heterolysis Carbocation Rearrangement Nucleophilic Additionarrow_forwardCan you work through the whole reaction and explain please?arrow_forward(Intermediate) Reactant Na+ + Tip: Only add curved arrows in this sketcher Apply Mechanism Hint Solution 1 remaining step(s) can be solvedarrow_forward
- Just problem number 2 please. Thanksarrow_forwardDraw the complete, detailed mechanism (curved arrows) for the following reaction. Br 2. H₂O OKarrow_forwardWhich of the following is the rate-limiting step of a reaction? A) the fastest step in the reaction mechanism B) the slowest step in a reaction mechanism C) the last step in the reaction mechanism D) none of the abovearrow_forward
- Increasing the concentration of either of the reactants of an Sn2 reaction increases the rate of the reaction. The primary reason for this is that increasing the concentration increasesarrow_forwardThe rate of the reaction with methanoic acid is greater than the rate of the reaction with ethanoic acid.Explain why. You should refer to ions in your answerarrow_forwardplease provide mechanismarrow_forward
- Draw the complete mechanism and predict the products of the following Cl2 Fe or FeCl3arrow_forwardRank the following substrates in order from slowest Sy2 reaction rate to fastest. Br H3C Drag answer here fastest Br Drag answer here second slowest Br Drag answer here slowest Br second fastest Drag answer herearrow_forwardPlease draw the chemical mechanism for Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY