
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
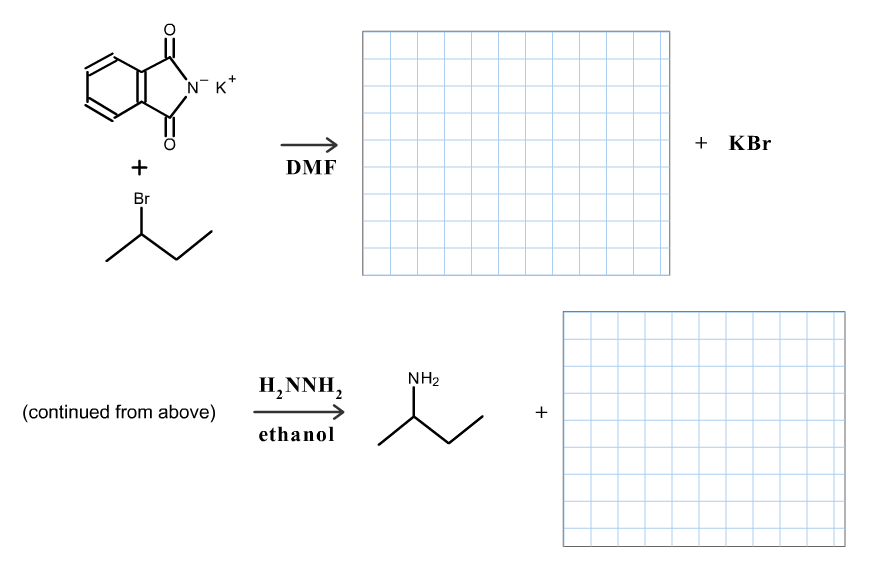
Transcribed Image Text:`NK*
+ KBr
DMF
Br
NH2
H,NNH,
(continued from above)
ethanol
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- 1-41 Determine whether the following pairs of structures are actually different compounds or simply resonance forms of the same compounds. (a) and (C) -yo-y-0-0 ان 0- O-H and CH=C-H and CH-C-H ۔ 0-H (k) H-C-NH, and H-C=NH, and and (e) (g) CH=CH-CH, and CH-CH = CH, (h) CH=C=0 and H-C=C-OH 0 H 0-H +O-H (i) H-C-C-H and H-c=C-H (j) H-C-H and H-C-H ---......... H NH₂ +NH2 || (f) CH-C-NH and CH-C=NH an_e_ H-C=CH-CH₂ (1) CH-C-CH=CH, and CH-C=CH-CH,arrow_forward14. Which labeled proton is most acidic in the following Lewis structure?arrow_forwardWhich one is the correct representation of methane (CH4) in 3 D shape (using wedge-dash line model)? Η Η Η Η Ή H"Η Τ Οι ΟΙ Ο III ΟIV Η Η || -H IIIarrow_forward
- Help the other image shows the wrong answer. It's not either one together or individuallyarrow_forwardIdentifying organic functional groups compound || C CH3 - O family - - CH₂- CH3 ester CH3 | CH3 — O — C-H CH₁₂ 0 = C - CH₂- CH3 OH ☐ ☐ 0 = CH - CH2-CH3 ☐arrow_forward= O PRINCIPLES OF ORGANIC CHEMISTRY Identifying rigid parts of an acyclic organic molecule In the actual molecule of which this is a Lewis structure, which of the labeled distances can change? marked 10 H O H B G1C F H A N E unmarked C O H List all the distances that can change. For example, suppose all the distances were measured at a certain time, and again 0.1s later. If distance A might be 50% bigger or smaller the second time, but all the other distances are certain to be the same, you should write "A". If A and B might be different the second time, but no other distances, you would write "A, B". And so on. Note for advanced students: you can assume the molecule is dissolved in an appropriate solvent at room temperature. 00.... X 90/5 You can click the "unmarked" tab to see the molecule without any of the distances marked. 5 Jaarrow_forward
- Given below are condensed structural formulas derived from the same molecular formula (C,H14). Identify which pair represents structural isomers, that is, different molecules. CH3 CH3 CH, CH3 CH, CH, CH-CH3 CH, CH-CH-CH3 CH3 CH, CH-CH,-CH2 CH3 CH, CH-CH CH, CH3 CH3 A and C O Cand D O Band D O D and Aarrow_forwardFor each organic compound in the table below, enter the locant of the highlighted side chain. CH3 CH,— CH,— CH— CH, CH3 1 compound CH₂ | CH,—C− CH,—CH — CH CH3 モー CH3 CH,—CH— CH,—C— CH | CH₂ CH3 CH3 | CH3 locant of highlighted side chain 0 X Sarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY