
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Full form please
![CH₂ +
НО
Edy
Type of Reaction:
6. OH
7.
محمد
H
Type of Reaction:
+ [H]
8. For the following molecule, draw the monomers
H₂N
a. What byproducts (if any) would form during this polymerization?
b. Type of polymerization that occurred:
O
ZI
ZI
H
CH3
COOH](https://content.bartleby.com/qna-images/question/723ad50a-4242-4d1d-a859-ac2d9951eb02/8a1091ca-c4fc-43ac-ab56-90d1b5795e4d/az5vb5o_thumbnail.png)
Transcribed Image Text:CH₂ +
НО
Edy
Type of Reaction:
6. OH
7.
محمد
H
Type of Reaction:
+ [H]
8. For the following molecule, draw the monomers
H₂N
a. What byproducts (if any) would form during this polymerization?
b. Type of polymerization that occurred:
O
ZI
ZI
H
CH3
COOH
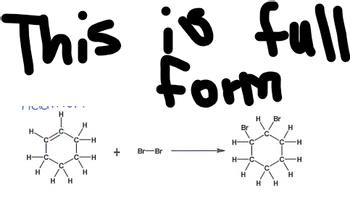
Transcribed Image Text:This is full
form
H
+
Br-Br
H
H
Br
-H
-H
Expert Solution
arrow_forward
Step 1: Introduce question
The question is based on organic reactions. We need to identify the product and explain its formation.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OH OH 1.OSO4 2. NaHSO3, H3O OH Select to Edit >arrow_forwardQUESTION 11 Write the condensed structural formula for the following molecule H. H. C N- H. For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). B IUS Paragraph Arial 10pt A Ix x X, 次 T T ΩΘ Click Save and Submit to save and submit. Click Save All Answers to save all answers. MacBook Pro !! !!! +]arrow_forwardTake Test: F23 Virtual Exam 1-2 x 4 ez Launch Meeting - Z... Grammar Help Co... edu/webapps/assessment/take/take.jsp?course_assessment_id=_2600851_1&course_id=2313023_1&content_id= 80124704_1&q LehmanQ- Lehman..... EasyBib®: Free Bibli... Esusu Remaining Time: 1 hour, 02 minutes, 15 seconds. *Question Completion Status: Question 4 R Moving to the next question prevents changes to this answer. New Tab Moving to the next question prevents changes to this answer. 9 How many total atoms are there in one formula unit of Pd3(PO4)4? 5 Q Search 6 Y & 7 U 4+ k 8 X 1 ( + 9 O O P Pearson Sign In O ( + [ DIYarrow_forward
- bard 1 ! New folder M Reading Mode: 1.5... M Gmail ض A- ش X Course Home Z~ is 2 https://openvellum.ecollege.com/course.html?courseld=17485264&OpenVellumHMAC-453c4e9377366ab5782f501c5246fbe0#10001 ▾ 2 Part A alt W= VA 3 Part B Complete previous part(s) S- = Part C Complete previous part(s) Part D Complete previous part(s) X a 2,4-floro, 5-methyl he XI Determine if each of the following cycloalkanes and alkenes can exist as cis-trans stereoisomers. Drag the appropriate items to their respective bins. Cis-trans isomers are possible. Submit Request Answer XO, F3 # 3 E- [» "! D[ LS ra. YouTube 4 C { $ R 24 F1 H₂C. Maps FS ▬▬▬ ▬▬ % 2,4-fluoro,5-methyl h X CH₂ 5 V} TY CH₂ ف GY Br. CH₂ 6 Ą Cis-frans isomers are not possible. XXX YI Copyright © 2022 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy Permissions | Contact Us | BY Y 5-Methyl-2,4-hexane X m.__ CH₂CH₂CH-CHF & 7 Hi FB U E W J NI Pearson ی 8 F9 DELL Reset Help Q trimethyl - prt sc 1 ÷ D MA ( 9 к. 30 F10 ن O…arrow_forwardhrome File Edit View History Bookmarks Profiles Tab Window Help 56% O Fri 4: St. John's University - My Appl x A ALEKS - Iffat Khan - Learn + A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFjmjhOdH_SyFuybOA6NIIU_F3qHYTiQI6Ca5ojhYwiUE.. O STOICHIOMETRY Finding mole ratios from chemical formulae This is the chemical formula for acetic acid (the chemical that gives the sharp taste to vinegar): CH,CO,H An analytical chemist has determined by measurements that there are 42. moles of carbon in a sample of acetic acid. How many moles of hydrogen are in the sample? Round your answer to 2 significant digits. mol Check Explanation O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privac étv IIarrow_forwardcan you submit a pdf? having trouble understanding thisarrow_forward
- Lable as true/false, if false change underline statementarrow_forwardAutoSave w Cinnamates DIR - Compatibility Mode OFF Home Insert Draw Design Layout References Mailings Review View Table Design Layout Tell me Share O Comments Times New... v 12 A A Аa v AaBbCcD AaBbCcDdE AaBb AаBbCcDdEe AaBbCcDdEe AaBbCcDdE E AaBbCcDdEe AaBbCcDdEe AaBbCcDdEe > Paste A • ev A No Spacing Title Subtle Emph... Normal Heading 1 Heading 2 Subtitle Emphasis Intense Emp.. Styles Pane B U v ab x, Dictate Sensitivity trans-cinnamic acid Work up your spectrum well, and fill in Table 1 below. Omit any impurities, solvents, or other elements that are not part of your compound. For the Figure, make a good drawing in ChemDraw (ACS-1996 settings), inserting the compound name in bold below the structure. Then using small, bold, lowercase letters to match the table, assign all of the 'H signals. Figure 1: Structure and 'H NMR assignments for trans-cinnamic acid. ОН Table 1: Experimental 'H NMR data for trans-cinnamic acid. Signal 8 (ppm) Mult. J-values (Hz) Int. a 11.3 1H b 1H 2H d 3H е…arrow_forwardthis question pleasearrow_forward
- W AutoSave On 2203 assignment - Compatibility Mode • Saved - O Search (Alt+Q) raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Times New Roman v 10 - A A Aa - A Normal Body Text List Paragraph No Spacing E Replace Paste В I U v ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard s Font Paragraph Styles Editing Voice Editor Reuse Files 1. Provide the missing reagents for each of the following reactions: CH2 но он ´HO CH,NH2 Ph CI Accessibility: Unavailable D Focus Page 1 of 19 259 words English (United States) 72% ENG 11:37 AM O Type here to search W 1°C Cloudy US 2022-04-20arrow_forwardA ALEKS - Dona Luc - Lean H My Grades - 2021 Spring Term (2 X Tutor.com Learning Suite M Hey - lucdona7@gmail.com - Gm x www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IBIZZhveDw7yX8A9043nt5P1XWJwAREDsbwIERg1UdvpRqH651Jk. O MATTER Solving applied density problems Mr. Auric Goldfinger, criminal mastermind, intends to smuggle several tons of gold across international borders by disguising it as lumps of iron ore. He commands his engineer minions to form the gold into little spheres with a diameter of exactly 6 cm and paint them black. However, his chief engineer points out that customs officials will surely notice the unusual weight of the "iron 3. ore" if the balls are made of solid gold (density 19.3 g/cm). He suggests forming the gold into hollow balls 3. instead (see sketch at right), so that the fake "iron ore" has the same density as real iron ore (5.15 g/cm). One of the balls of fake "iron ore," sliced in half. Calculate the required thickness of the walls of each hollow…arrow_forwardAutoSave Off CHML 1045 A6 Assignment (1) - Word O Search Danielle Hubbard DH File Home Design Layout References Mailings Review View Help A Share P Comments Insert Draw O Find - - 12 - A A Aav A 三 处T Arial AaBbCcDc AaBbCcDc AaBbCcI AaBbC AaBbCcC Replace Paste BIU - ab x, x A - er A I Normal T No Spac. 1 Table Pa. Heading 1 Heading 2 Dictate Sensitivity Editor Reuse A Select v Files Clipboard Paragraph Styles Sensitivity Reuse FilesA Font Editing Voice Editor L results. Molarity (M) of NaOH (from the bottle of NaOH): 0.204 mol/L Titration Number 3 4 34.44 mL 0.50ML Final Volume buret reading (mL NAOH) 34.00 33.85 mL 0.50mL 33.80 mL 0.50mL 0.50mL Initial Volume buret reading (mL NaOH) Volume NaOH used in titration (mL) = Final Volume buret reading (mL NAOH) - mL Initial Volume buret reading (mL NaOH) 33.94 For calculations multiply mL by 10-3 to convert mL toL Molarity (M or mol/L) NaOH from the bottle of NaOH 0.204 mol/L 10.0 mL 10.0mL 0.204 0.204 0.204 mol/L 10.0 mL 10.0 mL mol/L mol/L…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY