
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
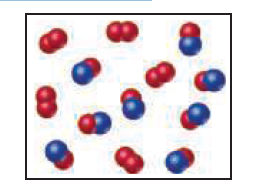
Nitrogen monoxide and oxygen react to form nitrogen dioxide.
Consider the mixture of NO and O2 shown in the accompanying
diagram. The blue spheres represent N, and the red ones
represent O. (a) How many molecules of NO2 can be formed,
assuming the reaction goes to completion? (b) What is the limiting
reactant? (c) If the actual yield of the reaction was 75%
instead of 100%, how many molecules of each kind would be
present after the reaction was over?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) How many moles of CO, contain 3.26x 10 molecules? 4.0 mol CO2 (b) What number of moles is equivalent to 7.17x10 atoms of Hg? 4.9 mol Hgarrow_forwardIf 35g of LiCl mixes with 25g of Ca(OH)2, (a) which is the limiting reactant? (b) what is the theoretical yield? LiCl + Ca(OH)2 → LiOH + CaCl2arrow_forwardBe sure to answer all parts. The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl₂(g) → 2AIC13(s) Assume that 0.42 g Al is mixed with 0.52 g Cl₂. (a) What is the limiting reactant? Cl₂ Al (b) What is the maximum amount of AlCl3, in grams, that can be produced? g AIC13arrow_forward
- Consider the haber-bosch process for the synthesis of ammonia from its elements. Calculate the theoretical yield in moles NH3 from the complete reaction of 15.6 grams H2 in the presence of excess N2 gas according to the following balanced chemical equation. N2(g) + 3H2(g) -> 2NH3(g)arrow_forwardHow many grams of P2O5P2O5 are formed when 9.51 g 9.51 g of phosphorus reacts with excess oxygen? Show the unit analysis used for the calculation by placing the correct components into the unit-factor slots.arrow_forwardHome-Grown Example: Calculation of Mass-Related Concentrations (a) A solution is made by dissolving 1.675 mg of sucrose (C₁2H22011) in 0.01091 g of water. What is the mass percentage of each component of the solution? (b) A 1762-kg sample of groundwater was found to contain 2.443 µg of Fe²+. What is the concentration of Fe²+ in parts per billion?arrow_forward
- I have attached an image. Thank you.arrow_forwardIn a reaction to produce NaF, 3.50 g of HF and 1.26 of Na2SiO3 are reacted according to the following: Na2SiO3 (s) + HF (aq) →H2SiF6 (aq) + NaF (aq) + H2O (l) (a) Balance the equation. (b) Calculate the theoretical yield of NaF. (c) If 0.792 g of NaF is formed, calculate the percent yield.arrow_forwardA chemist invents a new combustion analysis instrument that uses less O2(g) which saves money on each analysis. The improvement is that instead of CO2(g) being formed and trapped, CO(g) is formed and trapped. H2O (g) is also formed and trapped when substances that contain hydrogen are analyzed.(a) The chemist places 2.184 g of an unknown compound of carbon and hydrogen in the new analyzer. 4.627 g of CO (g) and 1.795 g of H2O (g) are produced. Find the empirical formula for the compound.(Molar masses (g/mol): CO = 28.0104, H2O = 18.015) (b) Despite the best efforts of the chemist, the molar mass of the compound cannot be determined (using this method). Clearly explain why this prevents the determination of the molecular formulaarrow_forward
- In an industrial process, hydrogen chloride, HCl, is prepared by burning hydrogen gas, H2, in anatmosphere of chlorine, Cl2. Write the chemical equation for the reaction. Below the equation, give themolecular molar and mass interpretations.arrow_forwardGlucose, C6H1206, can be represented by the molecular model shown below. If 1.00 mol of glucoseis submitted to combustion analysis, how many moles of CO2 and how many moles of H2O wouldbe formed?arrow_forwardbalance the following equationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY