
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
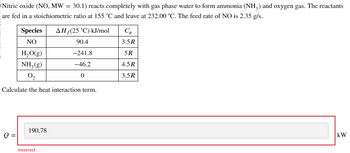
Transcribed Image Text:Nitric oxide (NO, MW = 30.1) reacts completely with gas phase water to form ammonia (NH3) and oxygen gas. The reactants
are fed in a stoichiometric ratio at 155 °C and leave at 232.00 °C. The feed rate of NO is 2.35 g/s.
Species
NO
AH (25 °C) kJ/mol
Ср
90.4
3.5R
H₂O(g)
-241.8
5R
NH2(g)
-46.2
4.5R
02
0
3.5R
Calculate the heat interaction term.
190.78
Q =
Incorrect
kW
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- The pressure of methane (CH4) and acetylene (C2H2) gas mixture in a container is 70.5 torr. This gas mixture is burned with sufficient O2 and converted to CO2 and H2O. The H2O and excess O2 in the environment are removed and only CO2 is left in the container. Since the pressure of CO2 is 96.4 torr at the same temperature, what is the mole fraction of acetylene in the starting gas mixture?arrow_forwardExample 3: A vessel content, one mole of carbon dioxide is heated. The carbon dioxide is dissociated 1 as: CO₂ → CO + 0₂. 2 If the equilibrium constant is given as: Kp = 30 ln(T) – 240. After equilibrium occurs, the carbon monoxide mole fraction is 0.098 find the reached temperature of the mixture.arrow_forwardQ2: Limestone at 25 °C is fed to a continuous calcination reactor. The calcination is complete, and the products leave at 900 C. Taking 1 metrie ton (1000 kg) of limestone as a basis and elemental species [Ca(s), C(s), O (g)] at 25 C as references for enthalpy calculations. prepare and fill in an inlet-outlet enthalpy table and prove that the required heat transfer to the reactor is 2,7 X 10 kJ. Heat CaCO3 (s) Ca0 (s) + CO2 (g)arrow_forward
- 29.2 Hydrogen sulfide (H₂S) is a common contaminant in natural gas. The dissolution of H₂S gas in water is a linear function of partial pressure, as is described by Henry's law of the form PA = H x. Values of H vs. temperature are provided below: T (°C) H (atm) mole H₂S/ mole MEA 20 483 PA (mm Hg) 30 449 Given the relatively low solubility of H₂S in water, an amine- based chelating agent is added to the water to improve the solubility of H₂S. Equilibrium distribution data for H₂S in a 15.9 wt% solution of monoethanolamine (MEA) in water at 40°C is provided below: 40 520 50 577 0.000 0.125 0.208 0.362 0.643 0.729 0.814 0.00 0.96 3.00 9.10 43.1 59.7 106 Describe the effect of temperature on the solubility of H₂S gas in water. b. Prepare equilibrium distribution plots, in mole-fraction coordinates (y₁ -— XÃ), for the solubility of H₂S in water vs. H₂S in 15.9 wt% MEA solution at 40°C and 1.0 atm total system pressure. Comment on the relative solubility of H₂S in water vs. MEA solution.arrow_forward(d) Determine the weight percentage, wt%, of the solid solution and liquid solution of a Cu-Ni alloy with a composition of 21 % Ni 79 % Cu, at a temperature of 1050 °C. Use part of the Thermal Equilibrium Diagram for Copper (Cu)-Nickel (Ni) alloy, as shown in Figure 1. Clearly show all your calculations. Temperature "C Figure 1 Part of the Cu-Ni thermal equilibrium diagram (not to scale) 1050 11 21 29 % Niarrow_forwardP2arrow_forward
- The fuel in the form of biogas (65%-mol CH4 and 35%-mol CO2) is burned in a furnace. In order for combustion to take place stoichiometrically, calculate the air to fuel ratio (vol/vol) from this problem!arrow_forwardUse the information about three gases, with the properties below to answer the following questions. Nitrogen (N2) Oxygen (02) Argon (Ar) 93.0 °C 83.0 °C 73.0 °C M (kg/kmol) 28 32 40 Use the van der Waals equation of state and mixing rules to calculate the temperature where 100 kmol of a mixture of argon (10 mol%), nitrogen (45 mol%) and oxygen (45 mol %) would occupy 46.27 m³, at a pressure of 6.28 MPa. It is known that the van der Waals constant for nitrogen are a=1.370 atm(m³/kmol)² and b=0.0387 m³/kmol, and for oxygen are a=1.382 atm(m³/kmol)² and b=0.03186 m³/kmol. 63.0 °C Tc (K) 126.2 154.6 150.8 Pc (atm) 33.5 49.8 48 @ 0.04 0.021 -0.004arrow_forwardA thermophilic organism can also obtain energy through alcoholic fermentation according to the equation (same as above): C6H12O6(s) -→ 2C2H5OH() + 2CO2(g) Using the information below, calculate the most exact molar Gibbs energy (A,G") for the reaction at 95.0°C. (to save time, it is OK to transfer some values calculated from problem 8) Compound C.H1:O6 (s) CH$OH () CO2 (g) A,G° (kJ/mol) A,H° (kJ/mol) -1,273.3 S° (J/mol-K) Cp (J/mol-K) -910.6 210.3 219.2 -174.2 -277.0 161.0 112.3 -394.4 -393.5 213.6 37.10arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The