
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
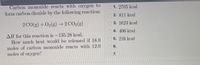
Transcribed Image Text:Carbon monoxide reacts with oxygen to
form carbon dioxide by the following reaction:
O 1. 2705 kcal
2. 811 kcal
2 CO(g) + O2(g) –→ 2 CO2(g)
3. 1623 kcal
O 4. 406 kcal
AH for this reaction is -135.28 kcal.
How much heat would be released if 18.0
moles of carbon monoxide reacts with 12.0
moles of oxygen?
O 5. 216 kcal
6.
O 7.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Balance the following redox reaction in an acidic solution: UO22+(aq)+Zn(s)->U4+(aq)+Zn2+(aq)arrow_forward1. An impure sample of compound A is contaminated with two impurities B and C. The sample is to be purified by recrystallization using ethanol as the solvent. The solubility properties of the three components are summarized below. Solubility in Solubility in ethanol Solubility in 50 mL Solubility in 50 mL ethanol at -78 °C at - 0°C ethanol at -78 °C ethanol at - 0°C (g) (g) Compound A 0.12 g/mL 0.02 g/mL Impurity B 0.58 g/mL 0.04 g/mL Impurity C 0.005 g/mL 0.0003 g/mL The impure (7.5 g) sample contains 5.0 g of compound A, 1.5 g of B and 1.0 g of C and is recrystallized using 50 mL of ethanol. The sample is boiled with 50 mL of ethanol, filtered by gravity and then cooled in ice and filtered by suction. a) How much compound A should be obtained as the final product? Will the sample be contaminated with any of the impurities? Explain (using calculations to support your answer-fill in the missing masses in the table above). Hint: For this question you should calculate the mass of each…arrow_forwardStandard Gibbs energy of formation, in kJ mol, at 25°C + 124.5 Benzene, C6H6(1) Carbon dioxide, CO2(g) Carbon monoxide, CO(g) - 394.4 – 137.2 Cyclohexane, C6H12(1) + 26.7 Ethanoic acid, CH3COOH (1) - 299.8 - 174.8 Ethanol, C2H$OH(1) Water, H20(1) - 237.1 1. Calculate the standard Gibbs energy change for the following reactions at 25°C: (а) С>H:ОН() + O2(g) > CH-СООН() -+ H2O(1) (b) 2 CO2(g) → 2 CO(g) + O2(g) Which of the above reactions, as written, is spontaneous at the standard state? [- 362.1; +514.4]arrow_forward
- (a) Consider the following chemical reaction: 3FE203(s) + CO(g) → 2Fe304(s) + CO2(g) AH° = ? Use the following thermochemical data to determine the enthalpy change of this reaction. (6) 2FE2O3 (s) AH° = -1652 kJ 4Fe(s) + 302(g) C(s,graphite) + ½O2(g) → CO(g) 3Fe(s) + 202 (g) → CO2(g) AH° = -110.5 kJ Fe304(s) C(s,graphite) + O2(g) AH° = -1117 kJ AH° = +395.5 kJ (b) What is the standard enthalpy of formation of carbon dioxide? (2)arrow_forwardAcetylene (C2H2) may be formed from methane (CH4) by pyrolyzing-decomposing at high temperature according to the reaction: 2CH4 (g) → C2H2 (g) + 3H2 (g) In a commercial reactor system, the methane is supplied as a liquid at 25°C to a heater where it is heated and vapourised leaving as a vapour at 650°C. The vapour then passes to the catalytic reactor in which a conversion of 40% is achieved. It may be assumed that there are no other reactions, that operation is at a pressure of one atmosphere and that the stream leaving the reactor is all in the vapour state. With a complete block diagram of the process, determine the heat transfer rate (kW) required to the reactor if it is operated isothermally. Evaluate on the value of the heat transfer rate obtained from your calculation. Component State Δ?? °(kJ/mol) Cp (kJ/mol·K) Methane Liquid ----------- ---------- Gas -74.85 0.34 Acetylene Liquid ---------- ---------- Gas 226.75 0.42 Hydrogen Gas ---------- 0.059 The Cp…arrow_forward8. Consider the following chemical reaction: 7 4 HBr(g) + O2(g) → 2 H20(g) + 2 Br2(g) Identify the substance oxidized (SO), the substance reduced (SR), the oxidizing agent (OA) and the reducing agent (RA). SO: SR: OA: RA:arrow_forward
- Alumina from bauxite separation stage calculations A crucial step in the production of aluminum from bauxite ore is the separation of alumina from the remaining mineral impurities in the ore. In the Bayer process this is accomplished by treating bauxite with aqueous NaOH to produce NaAIO2 (alumina). NaOH(aq) + Al(OH)3(s) → NaAIO2(aq) + 2H2O(1) Since NaAIO2 is water soluble while the residual mineral constituents of bauxite are not, a separation can be achieved by allowing the minerals to settle out and decanting the aqueous solution of NAAIO2 and unreacted NaOH. In order to further recover any NAAIO2 entrained in the settled mineral solids, this "mud" is repeatedly washed with water and allowed to settle, and the wash water is decanted. The figure below shows one stage of this washing-settling process. Wash water Slurry - Open to air Mixer/Wash tank Setling tank Decanted solution System boundary Washed mud In this stage, a feed slurry consisting of 10 wt% solids, 11 wt% NaOH, 16 wt%…arrow_forwardThe reaction of carbon monoxide(g) with water(l) to form carbon dioxide(g) and hydrogen(g) proceeds as follows: CO(g) + H2O(l) ---> CO2(g) + H2(g) When 10.2 grams of CO(g) react with sufficient H2O(l) , 1.02 kJ of energy are absorbed .What is the value of H for the chemical equation given?arrow_forwardLabel the acid, base, conjugate acid and conjugate base in the following reactions: a.) NH3 (aq) + H2O (l) ⇌ NH3+ (aq) + OH– (aq) b.) H2S (g) + HF (aq) ⇌ F– (g) + H3S+ (g) c.) HCl (g) + CH3OH (g) ⇌ CH3OH2+ (g) + Cl– (g)arrow_forward
- Example 3: A vessel content, one mole of carbon dioxide is heated. The carbon dioxide is dissociated 1 as: CO₂ → CO + 0₂. 2 If the equilibrium constant is given as: Kp = 30 ln(T) – 240. After equilibrium occurs, the carbon monoxide mole fraction is 0.098 find the reached temperature of the mixture.arrow_forward4. At 1084 °C solid copper melts to form a liquid. The phase change can be described as Cu(s) → Cu(t). The density of solid and liquid copper are 8.96 g/cm' and 7.75 g/cm' respectively. The enthalpies of combustion of the solid and liquid are -156.06 kJ/mol and -167.92 kJ/mol respectively. The product of combustion of both the solid and liquid is CuO(s). Calculate the standard enthalpy changes for the fusion process in kJ/mol. Calculate the change in internal energy when the sample is under a pressure of 500 kbar. Cu(s) → Cu(e) Solid copper liquid copper enthalples of combustion 8.96 g/cm3 7.75 g 1cm² said: -156.06 kJ/mol liquid-162.42 kJ/molarrow_forwardA fuel is prepared by mixing 4 moles of argon (Ar, inert) with 12 moles of ethane (C2H6) and 6moles of propane (C3H8) and undergoes complete combustion in air according to the followingreactions:C2H6 + 7/2 O2 reacts to form 2 CO2 + 3 H2OC3H8 + 5 O2 reacts to form 3 CO2 + 4 H2OAir may be considered as 21 mol% oxygen (O2) and 79 mol% Nitrogen (N2).The average molecular mass of a mixture can be calculated as Maverage = Σ xi Mi where xi and Mi are molefraction and molecular mass of component i. Atomic masses: C = 12; O = 16; H = 1; N = 14; Ar = 40.Calculate:a. the theoretical air required to burn the fuel (in kg air per kg fuel) b. the mole fraction of CO2 on a wet basis in the flue gas if the fuel is burned with 30% excess air,assuming complete combustionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The