
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can the following molecules be distinguished by IR? If so, specify the differences.
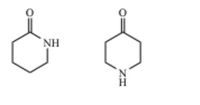
Transcribed Image Text:NH
Expert Solution
arrow_forward
Step 1
Ir stretching corresponds to different functional groups.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Need help with questions 4, 5, and 6. Please explain if you can. Thank you so much.arrow_forwardFor the Organic Chemistry topic of R and S, to make the structure R, does the prioritizing number have to be in order from 1 -> 2 -> 3 clockwise or can it be clockwise from 3 -> 2 -> 1?arrow_forwardWhich statement is true regarding [Fe(en)3]²+? The coordination complex has an optical isomer. O The coordination complex has no isomers of any type. O The coordination complex has a coordination isomer. The coordination complex has a geometric isomer. The coordination complex has a linkage isomer.arrow_forward
- please help with the bond line structures and IUPAC name The ones done in blue are what I've answered - please check those for correctness The others I need help with...arrow_forwardDetermine the correct hybridizaton (from left to right) about each interior atom in CH3CH2OH.arrow_forwardFor the molecule below, assign configurations (R or S) at C4, C5, and C6. C6 'OH OH C4 Но ОН C5 O C4: R; C5: R; and C6: R C4: S; C5: S; and C6: S C4: S; C5: R; and C6: R C4: R; C5: S; and C6: R C4: R; C5: S; and C6: Sarrow_forward
- Draw the skeletal (line-bond) structure of [CH₃CH₂CHC(O)CH₂CH₂CH₃]⁻. Include all lone pairs and charges as appropriate. What do the brackets and the - exponent mean for this formula?arrow_forwardGive detailed Solution with explanation needed..don't give Handwritten answer..show workarrow_forwardConsider the following four molecules, count how many sp2 atoms are conjugated how many are non-conjugated. Molecule A has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms. Molecule B has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms. Molecule C has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms. Molecule D has _ conjugated sp2 atoms and _ non-conjugated sp2 atoms.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY