
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Orgo Homework Help??
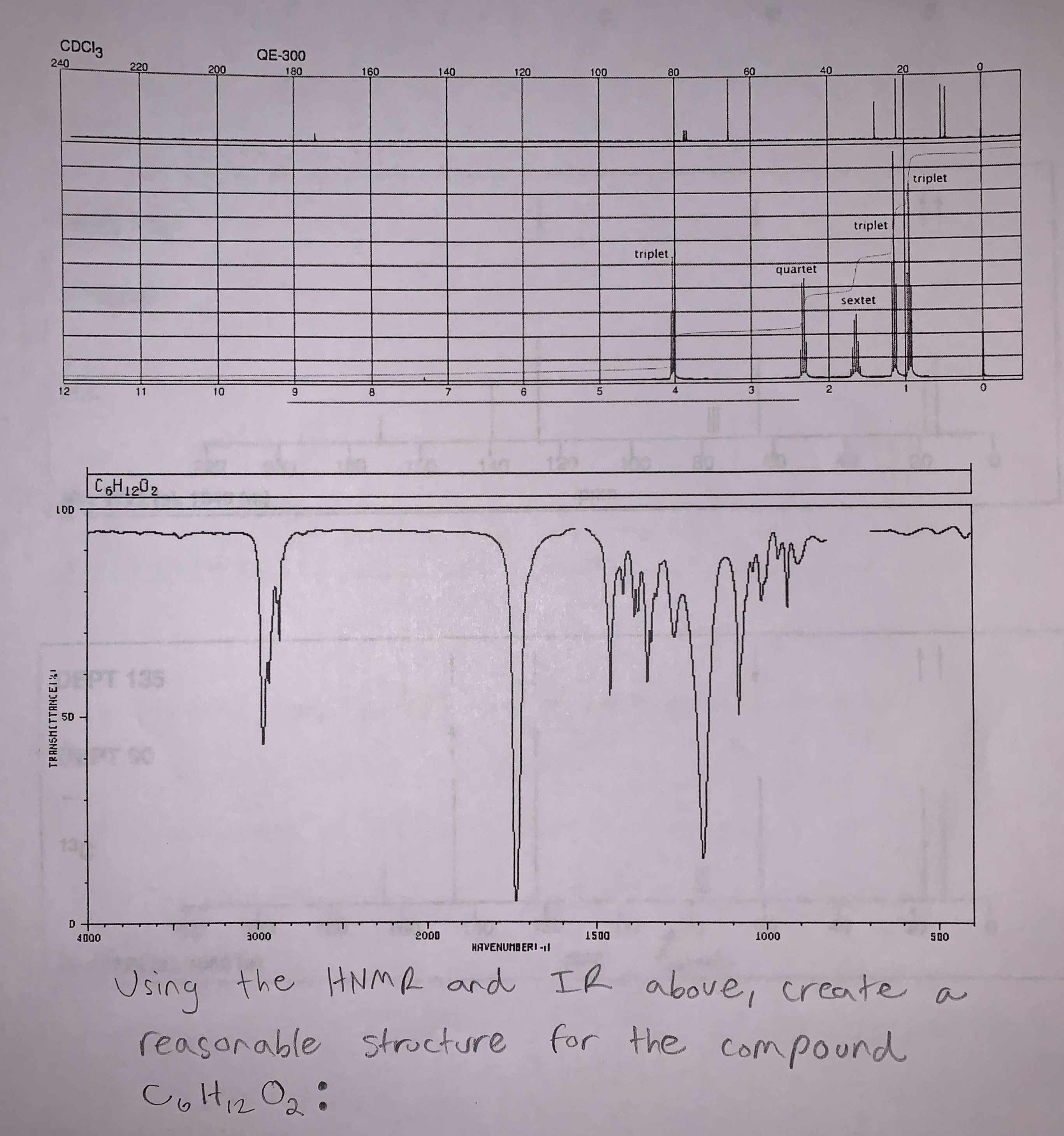
Transcribed Image Text:CDCI3
240
QE-300
220
200
20
40
180
140
60
160
120
80
100
triplet
triplet
triplet
quartet
sextet
0
2
3
12
5
7
11
8
C6H1202
LDD
DEPT 135
90
13
D
2000
1500
3000
1000
500
4000
HAVENUMBERI -Il
Using the HNM and LR
above, create a
strocture for the
reasanable
Compound
12
10
50
TRANSMETTANCEL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many liters of ethanol contain 1.10 kgkg of ethanol?arrow_forwardIS chemistry going to be funarrow_forwardA suspension of pediatric Tylenol is sold as a concentration of the active ingredient is 32 mg/mL. If the dosage is 0.125 g per 45-lb pound body weight, how much is necessary to give a child weighing 85.4 lbs.arrow_forward
- 5arrow_forwardPart A- Does a liquid release energy or absorbs energy when it changes to a solid?(think of when water changes to ice). A-release energy B-absorbs energy Part B- Absolute zero is the freezing point of water- 0 degrees Celsius and 0 degrees farenheight A-True B-Falsearrow_forwardThe maximum number of valence electrons that any element in the periodic table may have is eight. True or False?arrow_forward
- Phenobarbital 180 mg/m 2 /24 hours given every eight hours is ordered for a childwhose BSA (body surface area) is 0.29 m 2 . How many mg will you need if the child willbe on the medication for 10 days?arrow_forward2. Which of the following represent chemical processes? Which represent physical processes? a. Calcium chloride dihydrate (CaCl₂ *2H₂O) slowly heated in a crucible to become calcium chloride (anhydrous). b. A hydrocarbon such as propane (C,H₂) undergoes combustion to power a grill. c. A rock climber's rope becomes frayed and turns the color of the rocks. d. A dog urinates on an air conditioner coil and the coils become corroded.arrow_forwardA 145.3 mL sample of carbon dioxide was heated to 301 K. If the volume of the carbon dioxide sample at 301 K is 394.6 mL, what was its temperature at 145.3 mL?arrow_forward
- How much volume (in cm3cm3) is gained by a person who gains 12.6 lblb of pure fatarrow_forwardIn March 1989, the Exxon Valdez ran agroundand spilled 240,000 barrels of crude petroleum off the coastof Alaska. One barrel of petroleum is equal to 42 gal. Howmany liters of petroleum were spilled?arrow_forwardHuman blood typically contains 1.04 kg/L of platelets. A 1.85 pints of blood would contain what mass (in grams) of platelets? (1 gallon = 3.785 L, 1 gallon = 8 pints).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY