
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
7
Please help
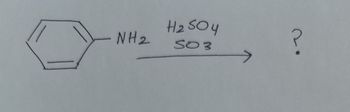
Transcribed Image Text:NH₂
H₂ SO 4
६OS
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Explain how the chemical formulas H for the same thing. and HO Can) are interchangeable notation (ag) Page 1 of 2 122 words P Type here to search DELL F9 F10 F11 F12 PriScr Ansert Delete F3 F4 FS F7 F8 %23 %24 Backspace 8arrow_forward22. Provided an acceptable name of the compound shown below. There is more than one correct answer. F Farrow_forwardplease help name the following using old naming systemarrow_forward
- Click the link to access the "Elements and Polarity" simulation by Concord Consortium. Generate an electron "surface view for the molecules below. You are able to rotate and resize the molecules in the simulation. Based on your findings, which molecule is most similar to hexane in terms of its polarity? O stearic acid O mineral oil O phospholipid ammoniaarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the products of the following reaction. Predict the reactants based on the intermediates provided. Include all lone pairs and charges as appropriate. Draw Reactant Br2 Br2 P Br: O Br: O Q >arrow_forwardWhich molecule is a complete organic molecule (i.e. all atoms are shown correctly)? A H. c=C–C= C–H H C H. C=C-C-C-H | H. H. C H. C- D H. нн . C С —С—Н C=C–C- C-H H H. ОН Н А I-0-I エーO I-Ú-I I-U-I I-U-I エー○arrow_forward
- 21. ADLC Assignment Booklet A3 Decide whether each statement is true (T) or false (F). Place your answer in the blank space given. Scientific theory states that the atoms in today's plastics were parts of microscopic plants and animals that lived in the oceans millions of years ago. b. Molecules of gasoline have more carbon atoms than do molecules of diesel fuel. c. Subjecting large molecules to cracking is useful because smaller molecules are manipulated more easily in a reaction to produce a desired product. d. Unsaturated compounds form during hydrocarbon cracking because too many hydrogen atoms are available for the number of carbon atoms.arrow_forwardName the following organic compounds: CH₂ CH₂ CH₂ CH3 CH₂- CH3- CH3 - - - CH₂ CH₂ CH3 - compound CH₂-C | CH₂ - CH3 - - — CH₂ - CH₂ - | I CH₂ CH₂ 1 - CH₂ 1 C 1 CH3 CH₂ CH₂ CH3 - — CH3 CH₂ CH₂- CH₂ CH₂ 1 + CH₂ - I CH3 - C CH3 name 1 0arrow_forward3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY