
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
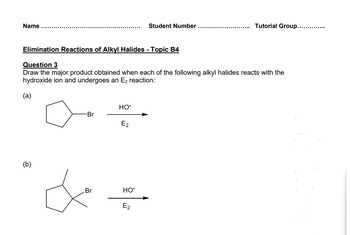
Transcribed Image Text:Name
Student Number
Tutorial Group..............
Elimination Reactions of Alkyl Halides - Topic B4
Question 3
Draw the major product obtained when each of the following alkyl halides reacts with the
hydroxide ion and undergoes an E2 reaction:
(a)
(b)
HO-
-Br
E2
Br
HO
E2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- H11.37 - Level 3 Unanswered • 3 attempts left What is the final, major product of this reaction? A a B b C C D d CH3 OMe 1) H3PO4 2) NaN3, acetone / H20 (50:50) ÕH Br a) OMe b) OMe Na N3 c) OMe b) OMe N3arrow_forwardThe reaction below could run through both substitution and elimination reactions. 1. Provide the correct reagent to produce the products shown 2. State which mechanism(s) was followedarrow_forward#9). Other picture is the instructions on how the arrows must be shown.arrow_forward
- 4. What is or are all the possible elimination product(s) for the reaction shown below? A) A B) A & B& C C) C & E D) A & C E) A & C & E (A) (B) OH (C) H₂SO4/ heat (D)arrow_forwardQuestion 1 pleasearrow_forwardMoving to another question will save the reporse Question 22 Using the pool of reagents provided below (table) complete the statement regarding the reaction below Fill in the blanks The following transformation can be accomplished by using the corresponding to the reagents from the reagents pool below set of reagents Write the letter in UPPERCASE AICHAL ZICul A) CH₂l2, Z(Cu) B) PhMgBr 2 H3O* C) H₂SO4, H₂O D) Na, NH3 E) Cl₂, CC, 1 mol F) HgSO4, H₂SO4, H₂O G) HBr, 1 mol H) B2, CC, 1 mol I) HCl, 1 mol J) Sia BH; 2) H₂O2, NaOH KI PhMaBrexcess 2) H.O K) PhMgBr excess, 2) H₂0* L) HCl, excess (M) Ch, CCI, excess N) HBr, peroxide O) Lindlar's catalyst P) Pd, H₂, ethanol Q) 03; 2) H₂O R) KF, Acetone S) KF, H₂O T) BHS. THF 2) H₂O2, NaOHarrow_forward
- What is the major product of the following series of reactions? The correct answer is A, but please explain why including the type of reactions and why the other options are incorrect.arrow_forwardDraw the major product obtained when the following alkyl halide undergoes an E2 reaction. If you expect no reaction to occur, submit the starting material as your answer. Interactive 3D display mode CH3 CI Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. + L H 2D EXP. CONT. H Carrow_forwardHW10 #14arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY