
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
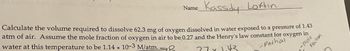
Transcribed Image Text:Name
Kassidy Loftin
-Partial
-mole
Fraction
Calculate the volume required to dissolve 62.3 mg of oxygen dissolved in water exposed to a pressure of 1.43
atm of air. Assume the mole fraction of oxygen in air to be 0.27 and the Henry's law constant for oxygen in
water at this temperature to be 1.14 x 10-3 M/atm.
77 x 1.43
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- The dispersed phase of a certain colloidal dispersion consists of spheres of diameter 1.0 102 nm. (a) What are the volume (V=43r2) and surface area (A = r2) of each sphere? (b) How many spheres are required to give a total volume of 1.0 cm3? What is the total surface area of these spheres in square meters?arrow_forwardA solution is made by dissolving 34.0 g of NaCl in 100 g of H2O at 0C. Based on the data in Table 8-1, should this solution be characterized as a. saturated or unsaturated b. dilute or concentratedarrow_forwardA solution is made by dissolving 0.455 g of PbBr2 in 100 g of H2O at 50C. Based on the data in Table 8-1, should this solution be characterized as a. saturated or unsaturated b. dilute or concentratedarrow_forward
- How many theoretical plates are required to produce a solution with a mole fraction of hexane greater than 0.90? Figure B A temperature-composition diagram for hexane (C6H14)-heptane (C7H16) mixtures.arrow_forwardIn the 1986 Lake Nyos disaster (see the chapter introduction), an estimated 90 billion kilograms of CO2 was dissolved in the lake at the time. (a) What volume of gas is this at standard temperature and pressure? (b) Assuming that this dissolved gas was in equilibrium with the normal partial pressure of CO2 in the atmosphere (0.038%, or 0.29 torr), use the Henrys law constant for CO2 in water to estimate the volume of Lake Nyos.arrow_forwardCarbon tetrachloride (CCl4) and benzene (C6H6) form ideal solutions. Consider an equimolar solution of CCl4 and C6H6 at 25C. The vapor above the solution is collected and condensed. Using the following data, determine the composition in mole fraction of the condensed vapor. Substance Gfo C6H6(l) 124.50 kJ/mol C6H6(g) 129.66 kJ/mol CCI4(l) 65.21 kJ/mol CCI4,(g) 60.59 kJ/molarrow_forward
- The vapor pressures of several solutions of water-propanol (CH3CH2CH2OH) were determined at various compositions, with the following data collected at 45C: H2O Vapor pressure(torr) 0 74.0 0.15 77.3 0.37 80.2 0.54 81.6 0.69 80.6 0.83 78.2 1.00 71.9 a. Are solutions of water and propanol ideal? Explain. b. Predict the sign of Hsoln for water-propanol solutions. c. Are the interactive forces between propanol and water molecules weaker than, stronger than, or equal to the interactive forces between the pure substances? Explain. d. Which of the solutions in the data would have the lowest normal boiling point?arrow_forwardA 1.00 mol/kg aqueous sulfuric acid solution, H2SO4,freezes at 4.04 C. Calculate i, the vant Hoff factor,for sulfuric acid in this solution.arrow_forward6-67 Calculate the freezing points of solutions made by dissolving 1.00 mole of each of the following ionic solutes in 1000. g of H2O. (a) NaCI (b) MgCI2 (c) (NH4)2CO3 (d) AI(HCO3)3arrow_forward
- The solubility of ethylene (C2H4) in water at 20 C and 0.300 atm pressure is 1.27 104 molal. (a) Calculate the Henrys law constant for this gas in units of molal/torr. (b) How many grams of ethylene are dissolved in 1.00 kg water at 20 C if the pressure of the gas is 500 torr?arrow_forwardAn aqueous solution containing 0.250 mole of Q, a strong electrolyte, in 5.00 102 g water freezes at 2.79C. What is the vant Hoff factor for Q? The molal freezing-point depression constant for water is 1.86C kg/mol. What is the formula of Q if it is 38.68% chlorine by mass and there are twice as many anions as cations in one formula unit of Q?arrow_forward6-111 As noted in Section 6-8C, the amount of external pressure that must be applied to a more concentrated solution to stop the passage of solvent molecules across a semipermeable membrane is known as the osmotic pressure The osmotic pressure obeys a law similar in form to the ideal gas law (discussed in Section 5-4), where Substituting for pressure and solving for osmotic pressures gives the following equation: RT MRT, where M is the concentration or molarity of the solution. (a) Determine the osmotic pressure at 25°C of a 0.0020 M sucrose (C12H22O11) solution. (b) Seawater contains 3.4 g of salts for every liter of solution. Assuming the solute consists entirely of NaCl (and complete dissociation of the NaCI salt), calculate the osmotic pressure of seawater at 25°C. (c) The average osmotic pressure of blood is 7.7 atm at 25°C. What concentration of glucose (C6H12O6) will be isotonic with blood? (d) Lysozyme is an enzyme that breaks bacterial cell walls. A solution containing 0.150 g of this enzyme in 210. mL of solution has an osmotic pressure of 0.953 torr at 25°C. What is the molar mass of lysozyme? (e) The osmotic pressure of an aqueous solution of a certain protein was measured in order to determine the protein's molar mass. The solution contained 3.50 mg of protein dissolved in sufficient water to form 5.00 mL of solution. The osmotic pressure of the solution at 25°C was found to be 1.54 torr. Calculate the molar mass of the protein.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning