
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
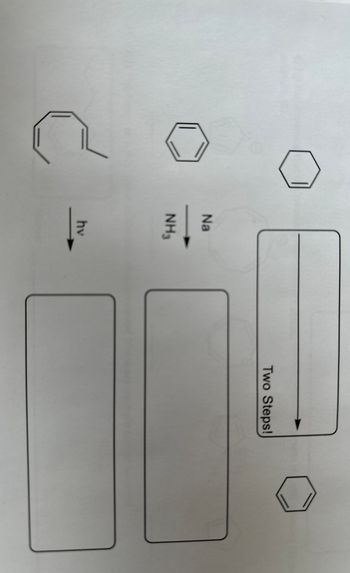
Transcribed Image Text:Na
NH3
hv
Two Steps!
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Pls show eork, will rate, D,E,Farrow_forwardDraw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H₂O or HC1. (You have 2 chances until the answer will be given; you have already tried 0 times) H3C CH 3 CI HN—CH3 C HỌC H C H CH3 H₂ Draw only one molecule in each drawing box.arrow_forwardWhich product is most likely to form in the reaction series depicted below? Br O₂N 1. HNO3. H2SO4 2. Br₂, FeBr3 A B Br Br Br NO2 D C E Brarrow_forward
- 1. The energy diagram of the following reaction is shown below: (CH3)3CBr + CH3COO- (CH3)3COCOCH3 + Br- A. Identify the reaction mechanism as indicated: 1. 2. 3. 4. 5. B. What specific type of reaction is represented by the given reaction? C. What is the order of the reaction as indicated by its kinetics? (please see picture below) 1. 4. 2._ 5. 3.arrow_forwardDrew the mechanismarrow_forward1A)Given the following value, tell whether the starting material or product is favored at equilibrium. Keq = 6 A. Starting material favored B. Product favored 2B) Given the following value, tell whether the starting material or product is favored at equilibrium. DG = 5 kcal/mol A. Starting material favored B. Product favoredarrow_forward
- What is the missing reactant in the following reaction? Select one: DA N OB. III O C. OD. II ? N&OC₂H₁ HOC₂H5 CHoli || & si "G OCH OCHSarrow_forwardClassify the reaction shown below.arrow_forwardSelect the incorrect reaction. NH2 1. NaNO2, HCI 2. CuCN a CN O b. NO2 NH2 Sn Oc Br H₂N Ph NaBH₂CN HCI Br HN Ph O d. се 忌 CN 1. KOH NH 2. Br Ph 3. KOH, H₂O 1. LAH 2. H₂O J H2N 'Narrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY