My answer is correct but I need more detail about my answer, so watch the professor's comment and write your solution more detail. I attach the question and the professor's comment. I attach my solution and comment
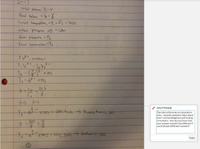
2. Consider two cylinders of gas identical in all respects except that one contains diatomic gas and the other a monoatomic gas. Both cylinders initially contain the same volume of gas at 0°C and 1 atm of pressure and are closed
by a movable piston at one end. Both gases are now compressed adiabatically to one-fourth their original volume.
(i) Which gas will show the smaller temperature increase? (Hint: Think about degrees of freedom.)
a. the diatomic gas
b. the monoatomic gas
c. Neither; both will show the same increase.
d. It is impossible to tell from the information given.
(ii) Which gas will show the greater pressure increase?
a. the diatomic gas
b. the monoatomic gas
c. Neither; both will show the same increase.
d. It is impossible to tell from the information given
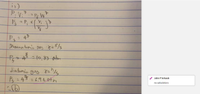
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

- For the diagram shown below, if an ideal gas transition from point A to point D, then: the Temperature of the gas sample will____? the Pressure of the gas sample will______?arrow_forwardNeed help, please. Suppose you have 8.4 L of an ideal gas at 25 °C initially in a container equipped with a piston. You arrange to keep the pressure on the gas equal to 2 atm as you compress the gas to a volume of 2 L. How much work does it take to compress the gas?arrow_forwardHelp me number 4 please do it step by step.and make sure that I can understand the words and symbols do you usearrow_forward