
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Musk ambrette is used as a perfume base to enhance and maintain the fragrance of perfume. Why is the Friedel-Craft connection done on the t-butyl group and not the methyl group? Write down reagent X !
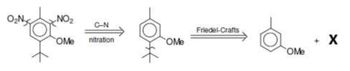
Transcribed Image Text:O₂N₂
NO₂
C-N
OMe nitration
OMB
Friedel-Crafts
OMe
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which ketone you think has the most stable π system? Explain your answer clearly based on the carbonyl grouparrow_forward1. Why does 1-butanol and cyclohexanol are immiscible whereas 2-methylpropanol and ethylene glycol are miscible? 2. What is the oxidation product of methanol inside the body and why is it harmful? 3. Write your observation based on the acidic properties of each sample Sample Observation 1-butanol 2-butanol 2-methyl-propanolarrow_forwardorganic chemistry reaction questionsarrow_forward
- explain why the name and draw the molecules of each please Please need both parts 4,5 Will provide helpful ratings for correct solution.arrow_forward22. The traditional reasoning for why phenol is more acidic than cyclohexanol resides in a resonance analysis. What is another reason that phenol is more acidic?arrow_forward- 17 Give the IUPAC name for the following compound: Br CO₂H A. 3-bromo-5-methylheptanoic acid B. 3-(1-bromoethyl)-4-methylhexanoic acid C. 3-bromo-4-ethylhexanoic acid D. 3-methyl-5-bromoheptanoic acidarrow_forward
- Explain why meta hydrogens in phenol are more deshielded than ortho and para hydrogen atoms.arrow_forwardInstructions: Give the IUPAC name for each compound. A. CH₂ CH₂CH₂CH₂CCH₂COOH CH₂ B. CH.CHCHCH.COOH a CH₂CH₂ C. (CH,CH,), CHCH,CH COOH Instructions: Give the structure corresponding to each IUPAC name. a. 2-bromobutanoic acid b. 2,3-dimethylpentanoic acid c. 2-ethyl-5,5-dimethyloctanoic acid d. 3,4,5,6-tetraethyldecanoic acidarrow_forwarda The functional group of a thiol is the SH (sulfhydryl) group. true O false b The parent name of a thiol is the name of the longest carbon chain that contains the SH group. true false The S-H bond is nonpolar covalent. true false d The acidity of ethanethiol is comparable to that of acetic acid. true false e Both phenols and thiols are classified as weak acids. true false Previous Nextarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY