
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
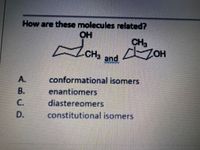
Transcribed Image Text:How are these molecules related?
OH
CH,
OH
CH
and
A.
conformational isomers
enantiomers
diastereomers
constitutional isomers
B.
C.
D.
PIT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- g. h. wit $ Ph Br li NaOCH, CH₂OH (cold, short time) 1. LDA 2 Br OCH₂ & 1. NaOCH₂ CH₂OH 2. BrCH₂CH₂CH₂ 3. H+, H₂O, heat 1. PPh 2. BuLi 3. NaOEt OEt EtOH NaOCH, CH₂OH (warmup, long time)arrow_forwardO Select the strongest acid. O I— ④SH HOH H OH Iarrow_forwardexe/lo_u-IgNslkr7j8P3ji Qs_dp5pR4ENzvdYC-70kXyMz36BqJhw3sVP80GQVcR8H8EYh1OU3pQMbCr ourse Home Login | Student Veri... Logout 5 MyProgrammingLab Imported From IE O ITE MEASUREMENT AND MATYER Predicting and naming ionic compounds formed by two elements Decide whether each pair of elements in the table below will form an ionic compound. If t formed in the spaces provided. empirical formula of ionic compound element #1 element #2 Forms ionic compound? name of ionic compound lithium lodine yes O no calcium sulfur Oyes no fluorine sulfur yes cesium uniseube O yes noarrow_forward
- CGE 32023#/ Dr. W. Scott Pers... Part C m = Submit Part D ΔΗ = How many grams of MgO are produced during an enthalpy change of -232 kJ ? Express your answer in grams to three significant figures. 0/ IVD| ΑΣΦ Submit Provide Feedback Krause etal_2007... Paraphrasing Paraphrasing Request Answer MacBook Air W aaac 2 0 0 ссссс VE ΑΣΦ Request Answer C 2. Ć Tool... Tool... ? How many kilojoules of heat are absorbed when 40.8 g of MgO(s) is decomposed into Mg(s) and O2 (g) at constant pressure? Lxpress your answer in kilojoules to three significant figures. 60 ? g X C C Ⓒ ✰ ✰ ☐ kJ Sentence Checker... | Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions Contact Us | + Update Next >arrow_forwardX, Mass of sample (g) = 0.7 Y, Mass of benzoic acid (g) = 0.3 Z, Mass of pellet (g) = 0.9 calculate the follwing below information Mass of sample in pellet = Mass of benzoic acid in pellet =arrow_forwardHow did you calculate frquencyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY