
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
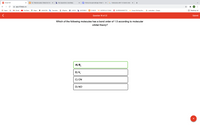
Transcribed Image Text:**Question 16 of 22**
**Which of the following molecules has a bond order of 1.5 according to molecular orbital theory?**
Options:
- **A) B₂⁻ (selected)**
- **B) N₂⁻**
- **C) CN⁻**
- **D) NO⁺**
This question tests the understanding of molecular orbital theory, specifically relating to bond order. Bond order is a concept that determines the strength and stability of a bond based on the number of bonding and antibonding electrons present in molecular orbitals.
In this question, you need to calculate the bond order for each given molecule or ion and identify which has a bond order of 1.5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the electron configuration of the ground state of the molecules/ions given below: Note: Draw the molecular orbital diagrams and write the electron distribution. Lizt - Bez - B22- - C2+ -arrow_forwardHow many molecular orbitals will be formed by the combination of the 2s and 2p atomic orbitals in 3.00 mol of atoms?arrow_forwardPredict the electron group geometry for a molecule whose central atom is sp3 hybridizedarrow_forward
- In each of the groups below, all molecules except one share the same orbital hybridization of the central atom. Identify which molecule is different, and name the hybridization for that molecule and the rest of the group. a) NO3", CO32", SO;2", BCI3 b) H2S, SIF4, SeO2, PB13 c) HCN, N2O, NO2", CS2 (Hint: N is the central atom in N2O) d) ClO3", NC13, AlCl3, H3O*arrow_forwardH 3 H H H C 0: H H :0: 8 H Aspirin (Acetylsalicylic acid) The hybridization of the atoms C-1, O-4, C-5, and O-8 in the above molecule are There are choose your answer... く There are choose your answer... molecule? choose your answer... unshared pairs of electrons present in the molecule? sigma (o) bonds and choose your answer... pi () bonds are present in thisarrow_forwardIndicate the difference between valence bond theory, and molecular orbitaltheory? Which theory is correct?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY