
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
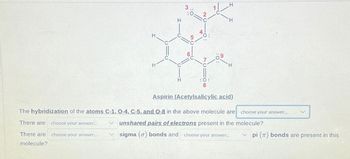
Transcribed Image Text:H
3
H
H
H
C
0:
H
H
:0:
8
H
Aspirin (Acetylsalicylic acid)
The hybridization of the atoms C-1, O-4, C-5, and O-8 in the above molecule are
There are choose your answer...
く
There are
choose your answer...
molecule?
choose your answer...
unshared pairs of electrons present in the molecule?
sigma (o) bonds and choose your answer...
pi () bonds are present in this
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- H3C Carbon a: Carbon b: H sp Carbon c: sp2 Carbon d: sp3 H H H₂C H₂ H₂ CH3 H c. H CH₂ mestranol OH CC=C-H CH₂ Determine the hybridization for each of the labeled carbon atoms: H₂arrow_forwardler the following list of molecules: XEF4 H2S NH2F CBr4 PCI5 H2CO NO3 BF3 CO2 IF2" В C G H. J You only need to provide a single answer for each question, even though in some cases chere may be more than one correct answer. Which of these molecules... a) ..has trigonal planar geometry? b) ...has a 90° bond angle? c)...has seesaw geometry? A.arrow_forward6.For the molecules shown below: L=molecule on left, R=molecule on the right H. CH3 at C H' ******* C%3Cd H -CH3 N. PCL R. (a) Add the lone pair of electrons to all atoms in both structures that have lone pairs (b) What hybrid orbital is used by the C in the CN group of L? (c) How many o (d) What is the approximate bond angle shown as “c" in L? - (e) What is the approximate bond angle shown as "f' in R? --- (f) What hybrid orbital is used by the O in the OCH3 group in R and how many t - bonds are there in L? --- -- -arrow_forward
- I have a question regarding hybrid vs atomic orbitals: 1) What would be the hybridizations of C and O in the molecule H2CO? It asks to draw an electron orbital diagram (with arrows) of all the electrons in C before and after hybridization. 2) Draw a diagram of the H2CO molecule showing the overlap of orbitals between the C and O atoms that make up the sigma (σ) and pi (π) bonds. Which orbitals make up the σ bond? Which ones form the π bond?arrow_forwardWhen forming a molecule, a central atom with this configuration will form what maximum 3pO00 3s N hybridization? O sp Osp O sp O sp² ssparrow_forwardConsider the following Lewis structure. (Lone pairs are not drawn in.) What is the hybridization of each C, N, and O atom? They have been labelled with subscript numbers for convenience. H-C,-H H-0,-Ċ2=C3=N,-H Iarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY