
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help with all parts
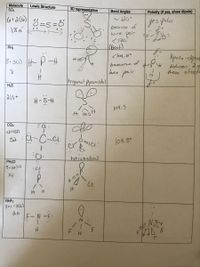
Transcribed Image Text:Molecule
SO2
Lewis Structure
3D representation
Bond Angles
Polarity (if yes, show dipole)
6+2(6)
8=s =ő
yes polar
because of
love pair
< 120
(Beit)
18é
PH3
Dpote -poe
between, dg
these stupte
< log,5°
5+ 3(1)
because of
lone pair
Priganal Pyrcmidal
H2S
2(1)+
H-S-H
109. S
It i90.5
कीट
32 : C-ci:
C4
109.5°
tetrahedral
PH2CI
5-1la)구
14
NHF2
5+1+7(2)
20
F-N-F:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Vmax and KM for the control using Line weaver-Burk curve.arrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help A 84% Wed 8:14 T WPAL 101_ 233 _Spring 2022 O NEW BEST Builder Hall 5 Base x ALEKS - David Teague - Learn x X Grades for David Teague: CHM A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZI6tTytly4Fcfu6zOtOf80MM9sfGh3NIP72XeoPmXkrk4zqM9ylm-ZZhvViL. O ☆ D Paused O CHEMICAL REACTIONS David Writing a chemical equation from a description of the reaction 2/5 Aqueous potassium nitrate (KNO3) and solid silver bromide are produced by the reaction of aqueous potassium bromide and aqueous silver nitrate (AgNO,). Write a balanced chemical equation for this reaction.arrow_forwardIf M=mass, L=length, and time = T; Dimension of kinematic viscosity is Select the correct response: [L^(2)]T [L^(2)][T^(2)] M/[L(^2)] [L^(2)1/Tarrow_forward
- rst.cuny.edu X o Mail-Chioma Egwuekwe - Outlo X W TEST 3 FALL 2022.doc ffice Editing for Docs, Sheets & Slides| L 2022.doc mat Tools Help Normal chrome-extension://bpmcpldpdmajfigpchkicefoigmkfalc/views/app.html Unsaved changes Times New.... 16 Edits will not be automatically saved. Save now BIU A- A + 8. A gas has an initial volume of 22.4 Liters at STP. What is its volume if the final pressure is changed to 2.0 atm and the temperature is changed to 373 K? 9. A gas has an initial volume of 40.0 Liters and an initial pressure of 1.0 atm. What is the final volume of the gas at the final pressure of 0.20 atmospheres? a. 20 Liters b. 200 Liters c. 160.0 Liters M 50 DELL Darrow_forwardGive clear handwritten Solution: Pressure of 50.0 psi is used to drive hexane through a 4.00 m length of capillary of 7.50 um diameter. Calculate the average flow velocity (v).arrow_forwardPlease help a bit unsure how to do itarrow_forward
- Crossword Puzzle 6 10 4 13 15 5 11 12 3 7 14 2 Name: 1 Across 1. Glassware. Erlenmeyer base, narrow opening. 3. Measure mass on a walks on a 4. If there's a outside in designated area. 10. You may not 11. Never beam. stock bottle. 7. Heating test tube over flame? Point opening from your face and others. 13. Always wear lab. 15. Dispose of 8. Spilled an irritant on skin or in eye? Flush for minutes. or drink in the lab. unused chemicals to a Down 1. The 2. 3. 5. 6. wide Gymnast exit quickly, and meet -toe shoes in the in the proper container. Before leaving the lab, area. hoods pull in noxious vapors. your work Glassware with a wide mouth, used to transport materials. Also a Muppet. Someone hurt? Notify your immediately. Pull back long. when working with flames. 9. Measure volumes with a cylinder. Also, what the class of 2023 did. 12. Always wear safety glasses or when anyone is working in the lab. 14. Be covered from shoulders to Also, "head, shoulders, and toes." 15. Trying to smell a…arrow_forward2- 3. Ch4 - Mastery Gmail с !!! X Canvas OWLv2 | Online teaching and l X docs.google.com/document/d/1TqDL3pNyiUclZ_7MKQ6brZDQFIAmOD1jfbX4SLstrel/edit Rangermail P University of Wisc... a se a A 100% Untitled document File Edit View Insert Format Tools Extensions Help 1 Normal text H Quizlet C chegg Verdana H I H₂N. I CI 1 I Untitled document - Google D X b Success Confirmation of Ques x + CH3 H I H 11.5 + H. H GPT P Student Center 2 -NH₂ Note that cis, trans isomers are an example of stereoisomers. Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. H CH3 H₂N- B I U A H I CI 3 ين - 4 Moncler NH₂| C + Pearson+ Public S... d = = ▶ Geo Book E Cengage Ochem 1 E EX 0 О н Update: ✓ Editing Share Harrow_forward3.Write in detail about working of any one hydraulic system application in construction area with neat diagram.arrow_forward
- The efflux time of glycerin sample is 320 seconds having a density of 1.26 grams per mL. This is based on the room temperature setting. Water is used as a standard with the computed efflux time of 225 seconds having a density of 0.997 grams per mL. Viscosity of water was identified as 0.7202 CP. What is the viscosity of glycerin?arrow_forwardFigure 8-5 х a А. quiz 8 figures Part... 1 page Figine 8.-5. continued E с. H + Ер нзот гхи prog СУИ prog. b H OA В. ET D. ея H₂Ö: H₂O: d о | Ⓡ Н гхи. pros -н my гхи progarrow_forwardChoose the best answer among the choices under each question/ statement. Thank youarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY