
Concept explainers
Molecule: 6-chloro-4-ethylhex-2-enoic acid
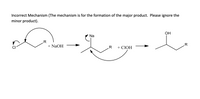
For the mechanism you will focus on the reaction of the portion of the molecule drawn only (not the R part). For any corrections, disregard the parts of your molecule represented by R-groups. Below the incorrect mechanism write a discussion (step-by-step) of the incorrect mechanism identifying all of the mistakes in the mechanism. Keep in mind that there will be more than one mistake in the given mechanism and that the product given is not necessarily the correct product of the reaction of your molecule with the given reagent. For each mistake, give a detailed, scientific explanation of why it is incorrect. Draw your own correct mechanism on a new page
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

- If the rate of reaction of [0.1 M] sodium cyanide with [0.1 M] 2- bromo-2-methylpropane is 1.2 mole/second, what would be the effect on the overall rate if the concentration of sodium cyanide is increased to [0.2 M] and the concentration of the alkyl bromide is decreased to [0.05 M]? H3C Br H3C CN + NaCN NaBr H3C CH3 H,C CH3 Provide a mechanistic explanation (using curly arrows) for the observed mixture of products in the following dehydration reaction (adding H2SO4), circle the major product, and state why you think it is the major product. OHarrow_forward6) The substitution reaction below to produce 1-iodobutane was performed using 1-bromobutane at a 0.1 M concentration and sodium iodide at a 0.1 M concentration. What would the rate law be for the reaction below? How would you expect the rate of the reaction to change if a 0.2 M concentration of sodium iodide was used? How would you expect the rate constant to change? Explain. Nal (0.1 M) Br acetone (0.1 M)arrow_forwardFurfuryl ethyl ether (FEE) was identified as a component that forms in aged beer, giving it a solvent-like taste U. Agric. Food Chem, 2004, 52, 1661) This ether can be formed from furfuryl alcohol (FALC, by either S,1 or S2 mechanisms. CH,CH,OH OCH,CH, furtury alcohol (prolonatec) furfuryl ethyl ether FEE) Draw a reasonable mechanism of the SN1 formation of FEE. Explain why it is possible for FALC to underga a SNI reaction, even though it would have to form a primary carbocationarrow_forward
- helparrow_forwardAnalyze the following reaction mechanisms. How many bond forming events occur in this mechanism? OH tot t + H₂O Acetone (CH3COCH 3) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b с d Three Two Four One :OH O.. :OHarrow_forward7. The reaction below is a very simplified version of a process involved in epoxy resins and adhesives, where an alcohol opens an epoxide (the three-membered ring). Explain why this ring opening is possible. Note: I'm not asking HOW, I am asking WHY. R-OH COO R-00 Harrow_forward
- Atom mapping, the determination of the fate of atoms of a starting material in the product, can help in the construction of a reasonable mechanism. Using the following labeling scheme, determine which atoms in the starting material (which are labeled with numerals) correspond to the atoms in the product (which are labeled with letters). 2 3 11 12 a 8. H2SO4 10 13 7 OH d f Reactant Product Reactant Product 1 8. 9. 3 10 4 11 5 12 13 7 2.arrow_forwardHow does the mechanism look like to get to this product? The molecular formula of the product of the following reaction is C6H11N.arrow_forwardWrite equations, with the necessary arrows, to illustrate the mechanism for the dehydration of tert-butyl alcohol. Describe each of the first two steps in the above mechanism in your own words. Step 1: Step 2:arrow_forward
- Use the two-step mechanism below to answer the following question. Step 1: NO (g) + N2O (g) → N2 (g) + NO2 (g) Step 2: 2 NO2 (g)→2 NO (g) + O2 (g) What is the intermediate? O N20 O NO O N2 NO2 O 02 MacBook Air DII 80 888 F6 F7 FB F3 F4 & %23 %24 3 4 5 8 E R Y F C в M I >arrow_forwardQuestion is attached.,.,arrow_forward3) Fill in the red box(es) with the missing reactant(s), reagent(s), product(s), solvent, and/or conditions and write a reasonable mechanism for the reaction. Not every box needs to be used and not every box necessarily corresponds to only a single species. If no reaction occurs OR no reagents exist to perform the reaction state, “NOT POSSIBLE” AND explain why. HO OH dil. H₂SO4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





