
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
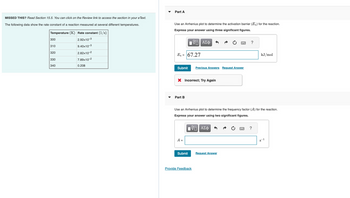
Transcribed Image Text:MISSED THIS? Read Section 15.5. You can click on the Review link to access the section in your e Text.
The following data show the rate constant of a reaction measured at several different temperatures.
Temperature (K) Rate constant (1/s)
2.92x10-3
9.40x10-3
2.82x10-2
7.89x10-2
0.208
300
310
320
330
340
Part A
Use an Arrhenius plot to determine the activation barrier (E₂) for the reaction.
Express your answer using three significant figures.
Π ΑΣΦ
Ea = 67.27
Submit Previous Answers Request Answer
X Incorrect; Try Again
Part B
A =
Submit
www.
Use an Arrhenius plot to determine the frequency factor (A) for the reaction.
Express your answer using two significant figures.
15| ΑΣΦ
Provide Feedback
Request Answer
?
wwwww
kJ/mol
?
8-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some measurements of the initial rate of a certain reaction are given in the table below. N2 H2 initial rate of reaction 2.31M 0.547M 89.0M/s 11.0M 0.547M 424. M/s 2.31 M 1.75М 911. M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k |I| x10 k = ?arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [H,] I initial rate of reaction 0.283 M 1.65 M 0.858 M/s 0.283 M 2.21 M 1.15 M/s 0.125 M 1.65 M 0.379 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [₂] [H₂] initial rate of reaction 0.912M 2.49 M 18.0M/s 0.374M 2.49 M 0.912M 11.2 M Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 2 significant digits. Also be sure your answer has the correct unit symbol. rate = k 3.03 M/s 364. M/s k = ☐ x10 ロ・ロ Xarrow_forward
- Some measurements of the initial rate of a certain reaction are given in the table below. [H] [1] initial rate of reaction 1.12M 2.03M 11.0M/s 1.12M 0.695M 3.77M/s 0.268M 2.0зм 2.63M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k|| x10 k =arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [H] 12| initial rate of reaction 1.04M 2.34M 17.0M/s 1.04M 1.10М 7.99 M/s 0.109 M 2.34М 1.78 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k I| x10 0 k =arrow_forward8. Smelling salt decomposes spontaneously at room temperature by the equation below. A student places a 10.00-g sample into a 1-liter sealed rigid vessel. What measurements should the student make to determine the rate of this reaction?(NH4)2CO3(s)→2NH3(g)+H2O(g)+CO2(g) a. change in mass and temperature b. temperature and time c. change in mass and time d. change in pressure and timearrow_forward
- General Chemistry 4th Edition McQuarrie • Rock • Gallogly University Science Books presented by Macmillan Learning The reaction C,H, (g) → 2 C,H,(g) has an activation energy of 262 kJ/mol. At 600.0 K, the rate constant, k, is 6.1 × 10-8 s-1. What is the value of the rate constant at 750.0 K? k = 1.34 x103 Incorrectarrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [N:] H2 initial rate of reaction 0.355M 2.28M 17.0M/s 0.355M 0.704M 1.62M/s 0.0958M 2.28M 4.59M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k|| x10 k =arrow_forwardDeducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. N2| H2 initial rate of reaction 圖 2.26M0.562M 9.00 × 10°M/s 2.26 M 0.108M 3.32 x 10ʻM/s 8.45 M 0.562M 3.37 x 10°M/s olo Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k| k = 1arrow_forward
- 9.arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. |H2|| ||2| | initial rate of reaction 1.50M| 2.25М 0.540 M/s 1.50M 6.45 М 1.55 M/s 5.57 М 2.25М 2.01 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k x10 k =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY