
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

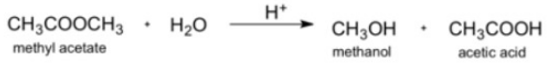
Transcribed Image Text:Metiyl acctate rcacts in acidic solution.
CH,COOCH,
• H30
CH,он . сн,соон
н*
methyl acetate
methanol
acesc acid
The rale law is first order in melhyl acelale in acidic solutiva, aad the rate coaslant al 25°C is 1.26 x 10*/8. Ilow long will it take for 63.0 of the melhyl acelale lo react?
Expert Solution
arrow_forward
Step 1
Given:
This reaction is first order reaction.
Rate constant of the reaction, k = 1.26*10-4/s
Percentage of methyl acetate reacts = 63%
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A(g) + B(g) ---> C(g) + D(g) <--- at constant temperature and pressure,an increase in the concentration of A(g) will cause ...arrow_forwardts/199084620/work Credit) | Lab: Reversible Reactions W 3) Matching. Reaction 2: Cobalt Chloride The color the COCI4+ 6H₂O Co(H₂0)6+²+4Cl 4) Choose the best answer. in the solution demonstrates that the equilibrium in this reaction is to ✓, causing a greater concentration of Co(H₂0)6+2 ions. Target due: Last Adding hydrochloric acid to the solution causes the equilibrium to shift to the CoCl2 because there is an increase in ▾ions. This gives the solution a color. Why does adding water cause a color change? O Water is a product of our original reaction, and when we added it, the concentration of the cobalt complex ion on the left side of the equation increased. Previous Sign out Next =arrow_forwardin the reaction 2SO(g) -- 2SO2(g), Kc= 4.0 x 10 -2 at 300 C. if the starting amount of SO2 was .5 M. what are the equilibrium concentrations of all species? (Hint use the calculator to find roots to the 3rd order equation)arrow_forward
- Withdr... Forms | Office of t... KB Viewing Your Aca.... Have Changes in... Scholarship Ameri... bio 1108 chat [Review Topics] [References] Use the References to access important values if needed for this question. M B # 3 Consider the reaction: P(s) + 3/2Cl₂ (g)=PC13 (g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K₁ and K₂, for the reactions below: 20 E D F3 P(s) +5/2C1₂ (9) PC15 (9) K₁ PCls (g) + Cl₂ (g) =PC15 (9) K₂ For answers with both a subscript and a superscript, enter the subscript first. For example, enter K2 If the first equilibrium constant should be squared. K = C Submit Answer $ 4 988 R F Show Hint % 5 Retry Entire Group 9 more group attempts remaining V Cengage Learning Cengage Technical Support FS T G A 6 MacBook Air B Y H & 7 U N * 0 * 8 J ► 11 FB ( M 9 T 1 K f O O H F10 L A P A command SAVAGE X FENT Next> Save and Exit F12 #1 A ? alt option delearrow_forward*00 FL 山 Rttps://WWW-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QldFNDUsAt... A O ADVANCED GENERAL CHEMISTRY 0/5 Calculating an equilibrium constant from an equilibrium. Hydrogen bromide and oxygen react to form bromine and water, like this: 4 HBr(g) + O,(g) → 2 Br,(g) + 2 H,0(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen bromide, oxygen, bromine, and water has the following composition: compound pressure at equilibrium HBr 76.6 atm 79.4 atm Br2 7.76 atm 5.82 atm o'H Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. Explanation Check O2022 McGraw Hill LLC. AllRights Reserved. Terms of Use Privacy Center Accessibility 9:37 3/18/2 DEC F5 F8 r pgDn. OL F12 PrtScr Insert Delete LL PgUp 24 Backspace Lock { [ Enter 4 Shift Alt Alt Ctrlarrow_forwardoms Date: 1) Cu(H2O)4+ + 4SCN Cu(SCN),+ 4H2O (a) Write the Kc expression for this equilibrium. (b) Would there be a shift to the right or to the left if KSCN solution was added to a solution containing this equilibrium? Explain your answer. 2) What is one of the major hazards associated with potassium dichromate?arrow_forward
- PC V ns ab AV H Slide Show Record Review View Help Α' Α' Α A O Search (Alt+Q) Font Aa Y !!! 年主持 Paragraph 1. Consider the endothermic reaction; JA [] √ D. RAISING THE TEMPERATURE HOMEWORK FOR CHEMICAL EQUILIBRIUM AND LE CHATELIER'S PRINCIPLE A. ADDING EXCESS C2H60 TO THE REACTION MIXTURE C. REMOVING C6H1202 AS SOON AS IT FORMS B. PERFORMING THE REACTION USING WET GLASSWARE C2H60 (1) + C4H802 (1) C6H1202 (1) + H20 (1) WHAT WILL BE THE EFFECT OF THE FOLLOWING ON THE EQUILIBRIUM (WILL THE REACTION SHIFT LEFT, RIGHT OR NO EFFECT. Shapes Arrange Quick Styles Chisom Obidiegwu : Notes Drawing 3 19 99+ 10. S 3 F HO 모 C [arrow_forwardne File Edit View History Bookmarks Profiles My Drive-Google X OKINETICS AND EQUILIBRIUM Setting up a reaction table initial 1 change Neuroscience Majo X C www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-lvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInICzlAf0OkuQp6C7cqj0bpH eGb9DAN.... bility and... 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po.... a SOLUTION: The le... Math 115 W-S Fa equilibrium Explanation e Q Neuroscience Pers X Suppose a 500. mL flask is filled with 0.50 mol of NO₂ and 1.5 mol of NO. This reaction becomes possible: 2NO₂(g) 2NO(g) + O₂(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O₂. You can leave out the M symbol for molarity. A 17,951 2 NO₂ 0 0 0 Check W S #3 NO 0 0 0 E D…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY