
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
- A reaction was run in a vessel attached to a manometer that initially had the same level of mercury in both tubes. Based on the change of in the level of mercury in the figure, which reaction must have occurred? Explain.
a) 2NaN3(s)à 2Na(s) +3N2(g)
b) Cl2 (g) + 2Br-(aq)à Br2(aq) + 2Cl-(aq)
c) C2H6(g) à C2H4(g) + H2(g)
d) H2(g) + I2(g) à 2HI(g)
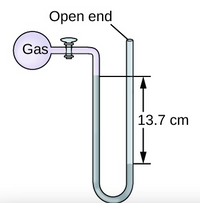
Transcribed Image Text:Open end
Gas
13.7 cm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which particle will conplete this reactionarrow_forwardS Eduphoria → CX Science Chemistry 4th NW DCA 22-23 | W COLOR THEME 13. Several JASON learning ambassadors for Aldine ISD recorded a demonstration for the students. They mixed two chemicals together and produced a 'golden rain'. The equation for the reaction is: KI + Pb(NO3)2 → KNO3 + PbI₂ The golden substance (image below) was found to be insoluble in water. Which of the following compounds is the precipitate? Q Q ZOOM < PREVIOUS 3 e d 8 C 9 10 t Oro 11 12 Oll y O 13 hp U O O O KNO3 CLEAR ALL ΚΙ O PbI₂ Answered 14 Pb(NC 15arrow_forwardIf this item does not open automatically you can open Data Sheet here = 106106334 Part I. Reaction of Fe³+ and SCN™ Test tube A B C 5 Adding NH3 (aq) 1/1 Action performed Experiment 15 Chemical Equilibrium and Le Châtelier's Principle Adding Fe(NO3)3 (aq) Adding NaSCN (aq) Adding NaNO3(aq) Part II. Reaction of Cu²+ and aqueous NH3 Action performed Mixing CuSO4 and NH3 (aq). Oll y 31 88% + Data Sheet Observations Observations. DELL العال[ O : * 8 CH127 Introductory General Chemistry Laboratory Conclusion Conclusion O Dec 18 8:09 2 3 Рarrow_forward
- Nitrate2arrow_forwardPLEASE HELP ME WITH BOTH AS They are part of 1 question and i really need help:( . I only have limited questions to ask so pls help asapp. Just a heads up, they are different questions tho but calculated together I will definitely consider giving thumbs up.arrow_forwardGiven the following equation: 2 PbS + 3O2 -----> 2 PbO + 2 SO2 What mass of O2 will react with 4.10 g of PbS?arrow_forward
- How many grams of water would be lost if a 8.000 gram sample of PtCl4·5H2O were heated?arrow_forwardWrite the net ionic equation for the reaction of copper(II) sulfate with sodium sulfide. A) 2 Cu* (aq) + SO,- (aq) → CU2SO, (s) B) 2 Na* (aq) + SO,²- (aq) → Na,SO, (s) C) Cu2* (aq) + S²- (aq) → CuS (s) D) 2 Cu* (aq) + S²- (aq) → Cu,S (s)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY