
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give typed solution not written
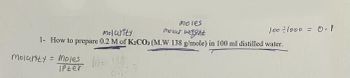
Transcribed Image Text:moles
molarity
molar weight
1- How to prepare 0.2 M of K₂CO3 (M.W 138 g/mole) in 100 ml distilled water.
molarity = Moles
IPter
100÷1000 = 0.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For Redox Analysis of Iron... ~0.02 M of potassium dichromate is prepared as titrant for dried unknown. ~.3 g of sample, 50ml of 6M HCl, 20mL SnCl2, 10 mL of saturated HgCl2 solution, 60 mL of 3M H2SO4, 15mL of concentrated H3PO4, 100mL DI water. HCl (+ heat in fumehood until samples dissolves), SnCl2 (added with pipet until solution changes from yellow to colorless/light green with 3 drops in excess), HgCl2 (after cooling), H2SO4, H3PO4, DI Water, is added to the sample solution with 8 drops of diphenylamine sulfonate indicator then titrated with the potassium dichromate to a violet blue endpoint. 4. Why is it necessary to carry out the reduction of iron and then the titration, before going on to the next sample 5. If you look carefully, there are pieces of tin metal on the bottom of the SnCl2 reagent solution. Why is it there?arrow_forwardProndetie Pnoduct CN CN Na eN. DMSO (B horeactienarrow_forwardCalculate the % relative error in solubility by using concentrations instead of activities for the Ag3AsO4 in 7.3 x 10 aAg = 0,25 a AsO4 = 0,4 alfa 0.05 M KNO3 (Ksp (Ag3AsO4) = -22arrow_forward
- Calculate the sum of coefficients for the following equation balanced for acidic conditions. XO4-2 + Y23+ → YO3+ + XO2-arrow_forwardCalculating Avogadro’s Number Using ElectrochemistryBackgroundAs discussed in lecture, electrochemistry allows stoichiometric calculations to be relatedto current and time, if a chemical equation that relates electrons to reactants and products isknown. Recall that electrolysis involves using an electrical current to decompose a compoundinto simpler substances. This particular experiment will involve the electrolysis of a sulfuric acidsolution to generate hydrogen gas. By measuring the amount of hydrogen produced by thecurrent and length of time of the electrolysis, it is possible to calculate a value for the Faradayconstant and thus also Avogadro’s number. The amount of hydrogen is determined by utilizingthe ideal gas law (PV = nRT) to determine the number of moles of hydrogen produced in theelectrolysis.The amount of charge that has passed through the solution is measured in coulombs(C). It is calculated by measuring the current (in amperes, A) that has passed through thesolution…arrow_forwardCalculating Avogadro’s Number Using ElectrochemistryBackgroundAs discussed in lecture, electrochemistry allows stoichiometric calculations to be relatedto current and time, if a chemical equation that relates electrons to reactants and products isknown. Recall that electrolysis involves using an electrical current to decompose a compoundinto simpler substances. This particular experiment will involve the electrolysis of a sulfuric acidsolution to generate hydrogen gas. By measuring the amount of hydrogen produced by thecurrent and length of time of the electrolysis, it is possible to calculate a value for the Faradayconstant and thus also Avogadro’s number. The amount of hydrogen is determined by utilizingthe ideal gas law (PV = nRT) to determine the number of moles of hydrogen produced in theelectrolysis.The amount of charge that has passed through the solution is measured in coulombs(C). It is calculated by measuring the current (in amperes, A) that has passed through thesolution…arrow_forward
- Write a balanced half-reaction for the oxidation of solid iodine dioxide (IO,) (10) to iodate jon in basic aqueous solution. Be sure to add physical state symbols where appropriate. Submit Assigni Continue O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy Acearrow_forwardWhich of the following metals will NOT dissolve in nitric acid or hydrochloric acid? Ag Cu Au Zn Ni Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Return to Assignment Provide Feedbackarrow_forwardFor Redox Analysis of Iron... ~0.02 M of potassium dichromate is prepared as titrant for dried unknown. ~.3 g of sample, 50ml of 6M HCl, 20mL SnCl2, 10 mL of saturated HgCl2 solution, 60 mL of 3M H2SO4, 15mL of concentrated H3PO4, 100mL DI water. HCl (+ heat in fumehood until samples dissolves), SnCl2 (added with pipet until solution changes from yellow to colorless/light green with 3 drops in excess), HgCl2 (after cooling), H2SO4, H3PO4, DI Water, is added to the sample solution with 8 drops of diphenylamine sulfonate indicator then titrated with the potassium dichromate to a violet blue endpoint. 3. How would you prepare a complete anlaysis procedure using KMnO4 as the oxidant instead of K2CrO7? Include sample and solution preparation, approximate weights of samples and reagents, procedure and chemical reactions. In particular what is the purpose of the Zimmerman Reinhardt Reagent?arrow_forward
- Why do both Reference and Indicator electrodes need in Potentiometric titration? answer at your own words ans answer to the point not any irrelevant wordsarrow_forwardCalculate the sum of coefficients for the following equation balanced for acidic conditions. XO93- + Y22+ → YO2- + XO2-arrow_forwardgine.html?ClassID=1563099459# Molten KF is electrolyzed by a current of 8.00 A for 18.5 hours. What mass of solid K is produced during this reaction? [?]gK Mass of K (g) Enterarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY